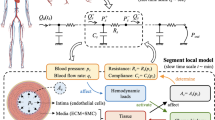
Abstract
Mechanical thrombectomy can be significantly affected by the mechanical properties of the occluding thrombus. In this study, we provide the first characterisation of the volumetric behaviour of blood clots. We propose a new hyperelastic model for the volumetric and isochoric deformation of clot. We demonstrate that the proposed model provides significant improvements over established models in terms of accurate prediction of nonlinear stress–strain and volumetric behaviours of clots with low and high red blood cell compositions. We perform a rigorous investigation of the factors that govern clot occlusion of a tapered vessel. The motivation for such an analysis is twofold: (i) the role of clot composition on the in vivo occlusion location is an open clinical question that has significant implications for thrombectomy procedures; (ii) in vitro measurement of occlusion location in an engineered tapered tube can be used as a quick and simple methodology to assess the mechanical properties/compositions of clots. Simulations demonstrate that both isochoric and volumetric behaviours of clots are key determinants of clot lodgement location, in addition to clot-vessel friction. The proposed formulation is shown to provide accurate predictions of in vitro measurement of clot occlusion location in a silicone tapered vessel, in addition to accurately predicting the deformed shape of the clot.
Similar content being viewed by others
References
Ajjan RA, Standeven KF, Khanbhai M, Phoenix F, Gersh KC, Weisel JW, Kearney MT, Ariëns RA, Grant PJ (2009) Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol 29:712–717. https://doi.org/10.1161/ATVBAHA.109.183707
Ashton JH, Vande Geest JP, Simon BR, Haskett DG (2009) Compressive mechanical properties of the intraluminal thrombus in abdominal aortic aneurysms and fibrin-based thrombus mimics. J Biomech 42:197–201. https://doi.org/10.1016/j.jbiomech.2008.10.024
Boodt N, Compagne KC, Dutra BG, Samuels N, Tolhuisen ML, Alves HC, Kappelhof M, Lycklama à Nijeholt GJ, Marquering HA, Majoie CB, Lingsma HF (2020) Stroke etiology and thrombus computed tomography characteristics in patients with acute ischemic stroke: a MR CLEAN registry substudy. Stroke 51:1727–1735. https://doi.org/10.1161/STROKEAHA.119.027749
Chueh JY, Wakhloo AK, Hendricks GH, Silva CF, Weaver JP, Gounis MJ (2011) Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. Am J Neuroradiol 32:1237–1244. https://doi.org/10.3174/ajnr.A2485
Di Martino E, Mantero S, Inzoli F, Melissano G, Astore D, Chiesa R, Fumero R (1998) Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: experimental characterisation and structural static computational analysis. Eur J Vasc Endovasc Surg 15:290–299. https://doi.org/10.1016/S1078-5884(98)80031-2
Duffy S, Farrell M, McArdle K, Thornton J, Vale D, Rainsford E, Morris L, Liebeskind DS, MacCarthy E, Gilvarry M (2017) Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J Neurointerv Surg 9:486–491. https://doi.org/10.1136/neurintsurg-2016-012308
Dutra BG, Tolhuisen ML, Alves HC, Treurniet KM, Kappelhof M, Yoo AJ, Jansen IG, Dippel DW, van Zwam WH, van Oostenbrugge RJ, da Rocha AJ (2019) Thrombus imaging characteristics and outcomes in acute ischemic stroke patients undergoing endovascular treatment. Stroke 50:2057–2064. https://doi.org/10.1161/STROKEAHA.118.024247
Feerick EM, Liu XC, McGarry P (2013) Anisotropic mode-dependent damage of cortical bone using the extended finite element method (XFEM). J Mech Behav Biomed Mater 20:77–89. https://doi.org/10.1016/j.jmbbm.2012.12.004
Fereidoonnezhad B, Naghdabadi R, Holzapfel GA (2016) Stress softening and permanent deformation in human aortas: continuum and computational modeling with application to arterial clamping. J Mech Behav Biomed Mater. https://doi.org/10.1016/j.jmbbm.2016.03.026
Fereidoonnezhad B, Naghdabadi R, Sohrabpour S, Holzapfel GA (2017) A Mechanobiological model for damage-induced growth in arterial tissue with application to in-stent restenosis. J Mech Phys Solids. https://doi.org/10.1016/j.jmps.2017.01.016
Fereidoonnezhad B, Dwivedi A, Johnson S, McCarthy R, McGarry P (2020a) Blood clot fracture properties are dependent on red blood cell and fibrin content. Acta Biomater (in press): https://doi.org/10.1101/2020.10.05.326165
Fereidoonnezhad B, O’Connor C, McGarry P (2020b) A new anisotropic soft tissue model for elimination of unphysical auxetic behaviour. J Biomech. 111, 110006. https://doi.org/10.1016/j.jbiomech.2020.110006
Gabbay JS, Zuk PA, Tahernia A, Askari M, O'Hara CM, Karthikeyan T, Azari K, Hollinger JO, Bradley JP (2006) In Vitro Microdistraction of Preosteoblasts: distraction promotes proliferation and oscillation promotes differentiation. Tissue Eng 12:3055–3065. https://doi.org/10.1089/ten.2006.12.3055
Gasser TC, Görgülü G, Folkesson M, Swedenborg J (2008) Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J Vasc Surg 48:179–188. https://doi.org/10.1016/j.jvs.2008.01.036
Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA (2018) Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg 10:34–38. https://doi.org/10.1136/neurintsurg-2016-012721
Johnson S (2020) Thrombus analogue material: Mechanical characterisation and use in in-vitro modelling of acute ischemic stroke treatment, (Doctoral dissertation, NUI Galway), http://hdl.handle.net/10379/16013
Johnson S, Chueh J, Gounis MJ, McCarthy R, McGarry JP, McHugh PE, Gilvarry M (2019) Mechanical behavior of in vitro blood clots and the implications for acute ischemic stroke treatment. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2019-015489
Johnson S, McCarthy R, Gilvarry M, McHugh PE, McGarry JP (2020) Investigating the mechanical behaviour of clot analogues through experimental and computational analysis. Ann Biomed Eng. https://doi.org/10.1007/s10439-020-02570-5
Kim J, Srinivasan MA (2005) Characterization of viscoelastic soft tissue properties from in vivo animal experiments and inverse FE parameter estimation. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer, Berlin, Heidelberg, pp 599–606
Krasokha N, Theisen W, Reese S, Mordasini P, Brekenfeld C, Gralla J, Slotboom J, Schrott G, Monstadt H (2010) Mechanical properties of blood clots - a new test method. Mechanische Eigenschaften von Thromben - Neue Untersuchungsmethoden. Materwiss Werksttech 41:1019–1024. https://doi.org/10.1002/mawe.201000703
Liebeskind DS (2005) Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am 15:553–573. https://doi.org/10.1016/j.nic.2005.08.012
Luraghi G, Rodriguez Matas JF, Dubini G, Berti F, Bridio S, Duffy S, Dwivedi A, McCarthy R, Fereidoonnezhad B, McGarry P, Majoie CB (2021) Applicability assessment of a stent-retriever thrombectomy finite-element model. Interface Focus 11:20190123. https://doi.org/10.1098/rsfs.2019.0123
Malone F, McCarthy E, Delassus P, Fahy P, Kennedy J, Fagan AJ, Morris L (2018) The mechanical characterisation of bovine embolus analogues under various loading conditions. Cardiovasc Eng Technol 9:489–502. https://doi.org/10.1007/s13239-018-0352-3
McEvoy E, Holzapfel GA, McGarry P (2018) Compressibility and anisotropy of the ventricular myocardium: experimental analysis and microstructural modeling. J Biomech Eng 140:081004. https://doi.org/10.1115/1.4039947
McGarry JP, McHugh PE (2008) Modelling of in vitro chondrocyte detachment. J Mech Phys Solids 56:1554–1565. https://doi.org/10.1016/j.jmps.2007.08.001
McGarry JP, Máirtín Ó, É, Parry G, Beltz GE, (2014) Potential-based and non-potential-based cohesive zone formulations under mixed-mode separation and over-closure. Part I: theoretical analysis. J Mech Phys Solids 63:336–362. https://doi.org/10.1016/j.jmps.2013.08.020
Moerman KM (2018) GIBBON: The Geometry and Image-Based Bioengineering add-On Software • Review • Repository • Archive. https://doi.org/https://doi.org/10.21105/joss.00506
Moerman KM, Fereidoonnezhad B, McGarry JP (2020) Novel hyperelastic models for large volumetric deformations. Int J Solids Struct 193–194:474–491. https://doi.org/10.1016/j.ijsolstr.2020.01.019
Monson KL, Goldsmith W, Barbaro NM, Manley GT (2003) Axial mechanical properties of fresh human cerebral blood vessels. J Biomech Eng 125:288–294. https://doi.org/10.1115/1.1554412
Nolan DR, McGarry JP (2016) On the compressibility of arterial tissue. Ann Biomed Eng 44:993–1007. https://doi.org/10.1007/s10439-015-1417-1
Pires PW, Dams Ramos CM, Matin N, Dorrance AM (2013) The effects of hypertension on the cerebral circulation. Am. J. Physiol. - Hear. Circ. Physiol. 304:H1598
Rouhani F, Fereidoonnezhad B, Zakerzadeh MR, Baghani M (2019) A computational study on vascular damage caused by shape memory alloy self-expandable and balloon-expandable stents in a stenosed artery. J Intell Mater Syst Struct 30:3113–3123. https://doi.org/10.1177/1045389X19880021
Saldívar E, Orje JN, Ruggeri ZM (2002) Tensile destruction test as an estimation of partial proteolysis in fibrin clots. Am J Hematol 71:119–127. https://doi.org/10.1002/ajh.10199
Slaboch CL, Alber MS, Rosen ED, Ovaert TC (2012) Mechano-rheological properties of the murine thrombus determined via nanoindentation and finite element modeling. J Mech Behav Biomed Mater 10:75–86. https://doi.org/10.1016/j.jmbbm.2012.02.012
van Dam, E. A., Dams, S. D., Peters, G. W., Rutten, M., Schurink, G. W. H., Buth, J., & van de Vosse, F. N. (2006). Determination of linear viscoelastic behavior of abdominal aortic aneurysm thrombus. Biorheology, 43(6), 695-707. https://content.iospress.com/articles/biorheology/bir439
Vande Geest JP, Sacks MS, Vorp DA (2006) A planar biaxial constitutive relation for the luminal layer of intra-luminal thrombus in abdominal aortic aneurysms. J Biomech 39:2347–2354. https://doi.org/10.1016/j.jbiomech.2006.05.011
Weafer FM, Duffy S, Machado I, Gunning G, Mordasini P, Roche E, McHugh PE, Gilvarry M (2019) Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. J Neurointerv Surg 11:891–897. https://doi.org/10.1136/neurintsurg-2018-014601
Zhao S, Gu L, Froemming SR (2011) Assessment of shape memory alloy stent deployment in a stenosed artery. Biomed Eng Lett 1:226–231. https://doi.org/10.1007/s13534-011-0036-5(2017)Abaqus2017.AnalysisUser’sGuide,DassaultSystèmesSimuliaCorp
Acknowledgements
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 777072.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Sensitivity analysis of the proposed model in terms of the isochoric and volumetric parameters in the unconfined compression test. Baseline parameters of \(D_{1} = 0.15, D_{2} = 0.6, E_{1} = 0.3 kPa, E_{2} = 9.0 kPa, D_{1v} = 0.01, D_{2v} = 0.05, \kappa_{1} = 2 kPa, \kappa_{2} = 20 kPa,\) have been used.
Influences of clot viscoelasticity on the occlusion location, \({\text{u}}/{\text{D}}\), in a tapered vessel. (A) Distribution of the maximum principal stress in clot at t=60 sec and t=200 sec, (B) Variation of the occlusion location with time for two loading profiles. The following parameters have been used: \(P_{0} = 75~mmHg,~~L/D = 2,~~\Phi = 5^{o} ,~~f = 0.2\), with the material parameters of the fibrin-rich clot from Table 1 .

reproduced from Johnson (2020).
Comparison of Ogden and proposed model with the in vitro experiments of occlusion of the platelet-contracted RBC-rich blood clot analogues (made from a 40% H clot mixture) in a silicon rubber vessel with taper angle of \({\Phi } = 0.9854^{{\text{o}}}\). (A) The location of clot lodgement (\({\text{u}}/{\text{D}}\)) and (B) aspect ratio of clot (\(\widehat{{{\text{AR}}}}\)). The material parameters from Table 1 have been employed. Experimental results
Appendix 1: Sensitivity analysis of the proposed model
Sensitivity of nominal stress and volumetric strain to the material model in the unconfined compression test is shown in Fig. 9. As shown in Fig. 9 (E–H), the stress–strain response is not sensitive to the volumetric parameters (\(D_{1v} , D_{2v} ,\kappa_{1} ,\kappa_{2}\)); however these parameters can control the volumetric strain (\(\varepsilon_{v}\)). On the other hand, as shown in Fig. 9 (C, D) the stiffness coefficients \(E_{1} ,{ }E_{2}\), affect both stress–strain and the volumetric behaviour.
Appendix 2: Constitutive models formulations and parameters
The model formulations and best-fit parameters for the hyperelastic models used in Sect. 2 are presented here. The strain energy density function for Ogden model is given as
where, \(\mu_{i} , \alpha_{i} ,\) and \(D_{i}\) are material parameters. The Yeoh strain energy density function is given as
where \(C_{i0}\) and \(D_{i}\) are material parameters; \(\overline{I}_{1}\) is the first deviatoric strain invariant defined as
The hyperfoam strain energy density function is given as
The best-fit parameters of the aforementioned models for the RBC-rich platelet-contracted clot analogous, corresponding to Fig. 1 (G, H), is presented in Table 3.
Appendix 3: Influence of the clot viscoelasticity
Recent experimental data show that thrombus material exhibits rate-dependent visco-hyperelastic behaviour (Johnson et al. 2020). To investigate the influence of viscoelastic behaviour of thrombus material on the occlusion location, we have used the Kelvin–Voigt model where the behaviour of the viscos element is implemented through the specification of a non-dimensional stress-relaxation curve, parameterised through a Prony series and the proposed hyperelastic model has been used for the elastic element. The dimensionless shear-relaxation modulus \(\overline{g}\left( t \right)\) and the dimensionless volumetric-relaxation modulus \(\overline{\kappa }\left( t \right)\) in Prony series are given as
where \(n\) is the number of the terms in the Prony series, \(\tau_{i}\) are the relaxation time constants for each term of the series, while the parameters \(g_{i}\) and \(\kappa_{i}\) sets the ratio of long-term to instantaneous effective shear and bulk modulus, respectively. A two-term Prony series is implemented with \(g_{1} = 0.15, \, g_{2} = 0.28, \, \tau_{1} = 60 \,{\text{sec}}, \tau_{2} = 500\, {\text{sec}}\) based on a previous study from our group.
In Fig. 10 two regimes of applied pressure are simulated: a single-step pressure increase, and a multi-step pressure increase. In both cases the clot eventually reaches the same final position. However, even for the case of the single-step pressure increase the clot does not reach its final position until ~400 s after the pressure application. This suggests that tapered tube experimental measurements should be executed over several minutes following pressure application. On the other end of the spectrum, this result suggests that in vivo a clot will reach its final occluded position in the vasculature at a relatively fast time-scale of several minutes, compared to the typical elapsed time (hours) prior to clinical intervention (e.g. thrombectomy).
Appendix 4: Convergence study
A typical mesh study as a proof of convergence of the results in terms of number of elements is shown in Fig. 11. The dimensions of clot and geometry of the tube are the same of the in vitro test (Sect. 3.3). The material parameters for the RBC-rich clot from Table 1 and friction coefficient of 0.09 have been used.
Based on the results in Fig. 11, the element size of 0.09 mm (33000 elements for this case) has been considered as the final mesh size and all simulations of the tapered tube in this paper have been performed with this element size.
Appendix 5: Comparison between the proposed model and Ogden model in taper tube test
We have simulated the tapered tube test by using the Ogden model, with the optimised material parameters in Table 3, for 5 different friction coefficients and the results are compared with the proposed model (Fig. 12). Ogden model was shown to replicate the stress–strain behaviour of clot with acceptable accuracy (Fig. 1G). However, the volumetric behaviour is a key determinant in tapered tube experiment and the proposed volumetric model improves the prediction of the results of tapered tube experiment, as demonstrated in Fig. 12.
Rights and permissions
About this article
Cite this article
Fereidoonnezhad, B., Moerman, K.M., Johnson, S. et al. A new compressible hyperelastic model for the multi-axial deformation of blood clot occlusions in vessels. Biomech Model Mechanobiol 20, 1317–1335 (2021). https://doi.org/10.1007/s10237-021-01446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01446-4