Abstract
Long held notions of the universally asocial octopus are being challenged due to the identification of high-density and interacting octopus populations in Australia, Indonesia, Japan and the deep sea. This study experimentally assessed the social tolerance and presence of potential prey items of Caribbean reef octopus, Octopus briareus, in a tropical marine lake (25°21′40″N, 76°30′40″W) on the island of Eleuthera, The Bahamas, by deploying artificial dens in multi-den groups or ‘units’ in the months of May and June 2019. Fifteen octopus were observed occupying dens (n = 100), resulting in 13 den units being occupied (n = 40). Two examples of adjacent occupation within a single den unit were identified but with zero examples of cohabitation/den sharing. Ecological models showed den and den unit occupation was predicted to increase with depth and differ between sites. Octopus also displayed no preference for isolated or communal units but preferred isolated dens over dens adjacent to others. Additionally, 47 % of occupied dens contained bivalve or crustacean items with no epifauna on their interior surface. The lack of epifauna suggests that these items have been recently ‘cleaned’ by occupying octopus and so represent likely prey. This study presents evidence of possible antisocial den use by O. briareus, a modification of the default ‘asocial’ ignoring of conspecifics typically attributed to octopus. This is likely in response to the high population density and may imply behavioural plasticity, making this system appropriate for further scrutiny as a research location on the influence of large, insular environments on marine species.
Similar content being viewed by others
Introduction
The social behaviour of animals regularly receives interest in the academic literature, exploring taxa across the animal kingdom (Economakis and Lobel 1998; Ritz et al. 2011) and the degree of interaction they have with conspecifics. Nearly all motile marine animals interact with other members of its species at some point in their lives, and some species aggregate together to improve feeding success (Gisburne and Connor 2015), protection against predators (Magurran 1990), and/or mate accessibility (Baeza 2008; Subramoniam 2013). Consequently, the commonly used definition of ‘social behaviour’ refers to all intraspecific interactions, from aggressive, through cooperative, sexual and parental (Rubenstein and Rubenstein 2013). An animal is, therefore, only being classified as ‘social’ if it displays cooperative group living (Rubenstein and Abbott 2017), with limited social behaviour between conspecifics the norm in many species. However, the ability to alter one’s behaviour in response to the conditions one experiences is a ubiquitous feature of animal behaviour and so it is sensible to consider such plasticity in a social context (Ruiz-Gomez et al. 2008; Oliveira 2012). The collective classification of taxa as non-social is, therefore, unrealistic and there is consequently a need to explore fringe populations in-situ to assess the varying degrees of interaction between animals before generalised comments can be made about the level of ‘sociality’ that a taxon expresses.
The lack of interaction between octopuses for example has led to a generalised view that these animals are solitary (Boal 2006) and averse to forming social aggregations, only interacting during mating (Huffard et al. 2008; Huffard et al. 2010) or conflict (Ibanez and Keyl 2010) as is the minimum for most species. Reports are available of octopus aggregations/clustering in select wild populations (e.g., Abdopus aculeatus—Huffard et al. 2008; Graneledone sp.—Drazen et al. 2003; Muusoctopus sp.—Hartwell et al. 2018; Octopus briareus—Aronson 1986; Octopus joubini—Mather 1982a; Octopus laqueus—Edsinger et al. 2020; Octopus tetricus—Godfrey-Smith and Lawrence 2012; Vulcanoctopus hydrothermalis—Voight 2005) but the drivers of these local events have been attributed primarily to habitat (Mather 1982a; Drazen et al. 2003; Scheel et al. 2017), resource availability (Huffard et al. 2008; Voight 2005), and relaxed predation pressure (Aronson 1986) rather than any social attraction (Mather and Scheel 2014). Consequently, the majority of studies establish octopus spatial distributions as widely spaced (Jereb et al. 2014), with individuals rarely coming in to contact with each other under typical conditions (Kayes 1974; Guerra 1981; Aronson 1986; Mather 1988).
However, a number of recent studies have identified active social interaction between octopus in the form of visual signalling, through body colour and pattern (Scheel et al. 2016; Huffard et al. 2008; Huffard et al. 2010), and non-aggressive physical contact (Caldwell et al. 2015; Edsinger et al. 2020). Conserved serotonergic signalling systems, similar to those that enhance prosocial human behaviours, have also been identified in the California two-spot octopus, Octopus bimaculoides (Edsinger and Dölen 2018), further weakening the assumption that all octopus are asocial.
The concept of the ‘asocial’ octopus derives from Mather (1982a)’s suggestion that the lack of territorial behaviour implicated by their generally dispersed spatial distribution, combined with rare examples of conspecific interactions, results in a taxon that is non-interacting. With this operational definition, Edsinger and colleagues (2020) clarified that if animals ignored one another when in close proximity, they could be thought of as asocial, whereas active avoidance of conspecifics would reveal an ‘anti-social’ predisposition. Moreover, the rapid establishment of dominance hierarchies based on size during laboratory experiments (Mather 1980; Mather 1985; Cigliano 1993) and during mating events in the wild (Huffard et al. 2010) implies that in response to increasing interactions with conspecifics, octopus are in fact capable of altering the degree of sociality they express (Edsinger et al. 2020) rather than being uniformly asocial. Behavioural plasticity of this form is not unreasonable when considering the high cognitive abilities displayed by octopus (though perhaps not the degree of ‘cognition’ reserved for mammals and birds—Emery and Clayton 2004; Mather and Dickel 2017). A plastic response to changing conditions is also in line with classic social behaviour theory (Hamilton 1964) that predicts that an animal will perform any one behaviour if the benefits to its inclusive fitness outweigh the costs of that behaviour. In a hypothetical scenario, the degree of social interaction or ‘tolerance’ towards conspecifics would then represent an energetic trade-off between population density versus a social-asocial-antisocial continuum, assuming that increased interaction with conspecifics occurs at higher densities. Following through on this hypothetical example, octopus appear to shift towards anti-sociality by avoiding neighbours in laboratory experiments (Cigliano 1993; Tricario et al. 2011) with increasing interaction rate.
Most social behaviour research in octopus has focussed on the use of shelters/dens as resource for octopus to occupy and compete over (e.g., Cigliano 1993). The use of dens stems from the ethical culturing of captive animals to minimise stress and cannibalism (Vidal et al. 2014; Fiorito et al. 2015) and the recognised importance of shelters for wild individuals. Dens are multi-functional for octopus, primarily providing anti-predator defence (Mather and Scheel 2014) with animals spending the majority of their time residing within them (Hartwick et al. 1984; Mather 1988; Forsythe and Hanlon 1997; Scheel and Bisson 2012). They consequently also facilitate safe egg-laying (Garci et al. 2016) and handling of prey. The latter is evidenced by the majority of prey being consumed in a ‘home’ den by the common octopus, Octopus vulgaris (Mather 1991), even if the prey was foraged a distance away. Due to this importance, octopus have been observed occupying a range of den types including rocky spaces (Anderson 1997), excavated holes (Guerra et al. 2014), discarded mollusc shells (Mather 1982b) and human litter (Katsanevakis and Verriopoulos 2004) such as glass bottles. In laboratory experiments, artificial dens are often deployed as a means to vary the relative quality of the resource available for octopus to occupy and provide an experimental factor (Cigliano 1993; Edsinger et al. 2020). The use of dens in this way has been pivotal in disentangling the apparent asociality of octopus populations but few attempts have been made to perform social behaviour experiments in natural environments.
To our knowledge, only four studies have explored in-situ artificial den enrichment as a technique to assess octopus social behaviour and den ecology (Voight 1992; Aronson 1986; Katsanevakis and Verriopoulos 2004; Mereu et al. 2018). These pieces of work highlight the preferential occupation of artificial dens by Caribbean reef octopus, O. briareus (Aronson 1986) and O. vulgaris (Mereu et al. 2018) and the active exclusion of conspecifics in a small radius, extending only a few centimetres, surrounding their den, (Aronson 1986). Aronson’s work is particularly important for research into the plasticity of social behaviour as it targeted a high-density sub-population of O. briareus within a tropical marine lake on the island of Eleuthera from The Bahamas. The high density of animals in the lake (known locally as ‘Sweetings Pond’) allowed the influence of population density on den preference, agonistic interactions and demography to be identified using a controllable resource in the form of artificial dens. For example, the observation that octopus did not occupy dens touching neighbouring dens (Aronson 1986) indicates that rather than being asocial, this sub-population is in fact antisocial at high densities; octopus chose to avoid dens that may contain conspecifics. Octopus density has been maintained in Sweetings Pond over the last 30 years (O’Brien et al. 2020) so there is potential to supplement this previous research and explore this finding using the same experimental den enrichment techniques. Rather than focussing solely on isolated den occupations and opportunistic sampling of neighbouring dens (Aronson 1986), there is a need to systematically enrich the population with den structures of varying den number, to test hypotheses of O. briareus den ecology and preferences in response to the possible presence of neighbours. It should be noted that the conditions experienced by O. briareus individuals within Sweetings Pond, and their consequent population dynamics and behaviour, are not representative of populations in the wider marine environment (Roper et al. 1984), but the confined nature of the Sweetings Pond ecosystem provides a unique opportunity to test the impact of insular marine environments on animals, as shown in other local species (e.g., lined seahorses, Hippocampus erectus—Masonjones et al. 2019).
This study aimed to investigate social tolerance in the Sweetings Pond O. briareus population, using den occupation preference as a proxy for social tolerance. Based upon the assumption that the predominantly asocial nature of octopus does not influence their preference for dens in close proximity to conspecifics (< 1 m), we hypothesize that isolated dens will be less frequently occupied over multi-den groups (dens in multi-den units will be occupied more frequently than isolated dens), due to there being a lesser quantity of den resource available. The non-occupation of dens in den groups compared to isolated would, therefore, indicate preferential occupation of isolated dens and antisocial behaviour rather than simply asocial (Edsinger et al. 2020). We then predicted possible ecosystem drivers of occupation events within the system using modelling techniques. In addition, the deployment of artificial dens provided an opportunity to quantify the potential prey item preference and handling behaviour of Sweetings Pond octopus.
Methods
Study area
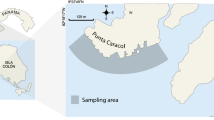
Sweetings Pond is a tidal anchialine lake (Fig. 1) located on the island of Eleuthera, in The Bahamas (25°21′40″N, 76°30′40″W). The ecosystem is isolated from the wider marine environment of the Great Bahama Banks, linked only by small aquifers in the porous limestone basin, which prevents the migration of animals, except as planktonic life stages. It is thought this ecosystem has been in isolation for a period of 10,000 years (Fleming et al. 1998; Bintanja et al. 2005; Masonjones et al. 2019).
Den enrichment experiments
While the Sweetings Pond population of O. briareus had previously been exposed to artificial den experiments (Aronson 1986), the ~1 year lifespan of the species (Hanlon 1977; Roper et al. 1984) results in a naïve population for this study. The dimensions selected for dens followed a den design that worked previously for this species and location: 31.2–60.8 cm length, 10.2 cm diameter and 5.1 cm entrance diameter (Aronson 1986). Dens were constructed from 4-inch (10.2 cm) PVC pipe cut to 41.5 cm length. This section of pipe was attached to two PVC end caps for a final structure of 50 cm long and 4090 cm3 volume. A 5.1 cm entrance hole was drilled into one of the end caps (Fig. 2a, b), as per the species’ preference (Aronson 1986).
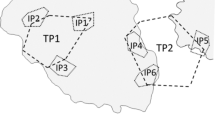
In this study, a ‘den’ is considered to be a single object available for an octopus to occupy, whereas a ‘den unit’ is a group of dens physically touching each other in a row (Fig. 2c), ranging in group size from one (i.e., a ‘den’) to four (a ‘den unit’). These den units acted as a single experimental replicate and aimed to vary the den resource quality from high (single/isolated den) to low (four abutting dens). One of each of the four described unit types were deployed per cycle, resulting, in four experimental units and a total of 10 individual dens available for occupation in a cycle.
The sites ‘Emmas’ (f.k.a. 'Octopus Den') and ‘Grouper Cave’ (Fig. 1) were subjected to five den deployment cycles at random locations within each site during the months of May and June 2019. A minimum distance of 50 m was maintained between units in a single deployment cycle to ensure independence of replicates (Supplementary Figure 1). Deployment GPS coordinates were randomly generated using the ‘random points in extents’ research tool in QGIS (QGIS Development Team 2019) and navigated to using a Garmin handheld GPS unit. ‘Emmas’ was selected to allow direct comparison to Aronson (1986), while ‘Grouper Cave’ provided a site with minimal human interference, due to its distance from the two public access points, and distinct benthic composition (O’Brien et al 2020). These historic site names refer to identifying landmarks or features of the site and have no reference to differences in habitat or other ecological differences.
Dens were deployed underwater for 4 days (n = 40) to allow sufficient time for octopus to encounter and engage with the novel den structures. Variation in soak time between dens was kept under 30 min. During deployment, dens were placed on the substrate along a north-south axis with the entrance hole facing the nearest shore to the east or west. Such placement minimizes the unintentional creation of quality differences among dens based upon the orientation of the unit. This precaution is necessary as no information is available on the influence of den position upon its ‘attractiveness’ to octopus. The complete unit was covered by sandbags to camouflage from predators and anchor the unit against hydrodynamic actions (Fig. 2c).
Den occupancy was assessed at the end of the 4-day deployment between 10:00 and 13:00. Assessment at this time ensured that the octopus population would be primarily residing within dens rather than actively foraging due to their nocturnal activity (Aronson 1986). During assessment, a free-diver filmed the interior, exterior, and sandbag surfaces of each unit for occupying octopus and other colonising species. The den was then transported to the surface, where a paddleboard acted as a floating platform. The interior and exterior were then re-filmed for non-octopus colonisers and any octopus unintentionally transported with the den photographed for size estimates. Occupying O. briareus were not handled but allowed to voluntarily return to the water to minimize stress. If more than one octopus was observed in a den unit, then they were considered either ‘adjacent occupiers’ or ‘cohabiting’ depending on their proximity. Adjacent occupancy refers to octopus occupying neighbouring dens in the unit, whereas cohabitation is the occupancy of two or more octopus in a single den.
Any potential prey items collected in the den were then photographed and catalogued. Sweetings Pond octopus have been reported to collect bivalves and block the entrance to artificial dens using dead bivalve shells, bivalve clusters and coral fragments (Aronson1986, 1989) and it is a behaviour displayed by the common octopus, Octopus vulgaris (Legac 1969). A number of species are reported to be predated on by the Sweetings Pond sub-population based upon midden and den discards: egg cockle, Laevicardiun laevigatum (reidentified as Ravenel's egg cockle, Laevicardiun pictum), Chione cancellata (reidentified as cross-barred venus, Chione elevata) and the crab Pitho aculeata (Aronson 1989). Other species are also postulated to be prey including the spotted dragonet, Callionymus pauciradiatus (reclassified as Diplogrammus pauciradiatus), the polychaete Eunice rubra, and an unidentified mysid shrimp after the observing of octopus foraging in the open (Aronson 1989). Items found within dens in this study were classified based upon two criteria; whether the item was alive/dead and ‘cleaned/uncleaned’. ‘Clean’ items were considered those with no epifauna on the interior surface of the bivalve shell/crustacean carapace, whereas ‘unclean’ exhibited epifaunal colonisation. Due to the prevalence of dead ‘calcareous rubble’ (i.e., the ‘unclean’ shells) on the lake’s bottom substrate, living bivalves regularly settle and attach to this calcareous rubble. These items may consequently be collected in the den as bycatch of true prey items or blocking material and so only ‘cleaned’ items can be considered recent prey following Scheel et al. (2017).
Chemical cues have previously been demonstrated to influence behaviour in octopus (Walderon et al. 2011; Morse et al. 2017), and so to mitigate bias related to possible odor-responses in future deployments, dens were emptied and cleaned with ethanol to remove biofilms and any residual O. briareus mucus. Sandbags could not be cleaned in this way so macrofaunal colonisers such as Atlantic pearl oyster, Pinctada radiata, C. elevata, and rough fileclam, Ctenoides scaber, were removed to standardize den quality with uniform sandbags, rather than deploy variably attractive habitats for octopus occupation.
To assess the benthic composition of the den deployment location, an image was captured from approximately 30–40 cm above the den unit. This distance provided a 50 cm ‘belt’ around the den unit. Twenty-five random points were generated in ImageJ (Rueden et al. 2017) using a custom macro, with the substrate at each point identified. If the point landed upon the dens themselves, the point was relocated to the nearest edge of the den and the substrate assessed there. From the substrate abundance data, percentage cover and Simpson’s diversity (Simpson 1949) were calculated for each den unit using the package ‘vegan’ (Oksanen et al. 2019).
Ecological survey
Using 30 m transects, invertebrate surveys were performed at five random points within each site. The survey’s orientation to the nearest shore was randomly assigned as ‘parallel’ or ‘perpendicular’, originating at the randomly generated coordinate. Surveys required the deployment of 1 m2 benthic quadrats at 5 m intervals either side of the transect line. Species present on the surface of the substrate were counted and measured. This process was repeated after the exposure of infaunal species by the fanning away of loose sediment, to minimize the underestimation of species’ abundance, particularly as many bivalves shallow-bury to escape predation (Peterson 1983; Zwarts and Wanink 1989).
Analytical methods
To predict the drivers behind O. briareus occupation, the octopus presence-absence data for a den unit, as well as for an individual den, were investigated across a range of ecologically relevant predictors. For den unit occupation, this was achieved using a non-nested generalized linear model (GLM) with an underlying binomial distribution (presence/absence) and logit link function. The ecological predictors initially explored as plausibly influencing occupation included site, month, depth (centered by subtracting the mean depth from absolute), benthic Simpson’s diversity, Mytilopsis sp. cover, P. radiata cover, calcareous rubble (CR) cover, sediment cover, den unit number (the number of dens available for occupation grouped in a unit), and distance to nearest public access point. Candidate models were identified using forwards and backwards stepwise regression based upon AIC, using the package ‘MASS’ (Venables and Ripley 2002). During this process, the variable ‘den unit number’ was anchored as a permanent predictor to allow the influence of den unit size to be estimated. The final model involved the fixed covariates, site (categorical variable with two levels: ‘Grouper Cave’ and ‘Emmas’), den unit number (categorical variable with four levels: 1, 2, 3 and 4), centered depth, and calcareous rubble cover (continuous).
A similar model was fitted for individual den occupancy presence-absence data using a generalized linear mixed effects model, with an underlying binomial distribution and logit link function, using the lme4 package (Bates et al. 2015). Den unit identity was defined as a random effect due to repeated measures of benthic characteristics and depth per unit and the interacting influence of adjacent dens on each other within the unit. All fixed covariates were retained from the den unit occupancy model.
The influence of each covariate is reported as odd ratios with confidence intervals calculated by exponentiation of the model’s coefficients. Odd ratios (OR) reflect the relative odds of the occurrence of an outcome (octopus occupancy here) given exposure to a predictor, compared to the odds of the outcome occurring in the absence of that predictor (Szumilas 2010), where an OR of 1 implies there is no association between exposure and outcome. In this study, an OR > 1 implies that exposure is associated with higher octopus occupancy, whereas an OR < 1 implies that exposure is associated with lower octopus occupancy. As the difference in ‘intervention’ between sites and number of dens within the unit are the primary predictors of interest, ‘Grouper Cave’, two-den unit size and centered depth were fixed as reference predictors for relative odds ratios to be calculated against. Therefore, statistical significance is determined by the exclusion of 1.0 from the 95% confidence interval for a predictor.
In the ecological data, items collected from dens differed in meeting assumptions of parametric testing. Thus, when comparing the availability of potential prey species between the two sites, a Student’s t test was applied to C. elevata and P. radiata abundance and a Mann-Witney U test was applied to Mytilopsis sp. abundance.
Results of statistical tests were considered significant at p < 0.05 with all analyses performed in R (R Core Team 2018). Assumptions of normality were tested using Shapiro-Wilk tests and equal variances with Levene’s tests. Due to the prevalence of zeroes in the dataset, as is common in the sampling of natural populations, the quality of all model residuals was assessed using the DHARMa package (Hartig 2020), with an excess of zeroes in a model’s residuals leading to its rejection.
Results
Octopus briareus den preference
Fifteen octopus individuals were observed occupying the interior or exterior of deployed den units. This resulted in an occupation rate of 15 out of 100 dens (15%) or 13 out of 40 units (38%) with two examples of adjacent occupation. Zero examples of cohabitation were observed. In both examples of adjacent occupation, the two octopuses were occupying adjacent dens within the overall unit (see Fig. 3 for an example) rather than dens furthest apart. Octopus presence was highest in one-den units (five occupations) over two- (three), four- (three) and three-den units (two occupations—Table 1). At ‘Grouper Cave’, ten octopuses were observed across 50 den and 20 den unit deployments compared to five at ‘Emmas’. A depth profile is provided of the occupation rates versus benthic composition in each site (Fig. 4).
Occupation behaviour of den units by O. briareus differed in response to site (i.e., the Odds Ratio, of observing an octopus; OR = 0.038, p < 0.05), with occupation less likely in ‘Emmas’ than ‘Grouper Cave’. Octopus briareus were also more likely to be encountered in deeper dens (OR = 2.426, p < 0.01). Calcareous rubble cover and den unit size (Table 2) had no significant effect on the occupancy probability of a den unit. As den unit number had no significant effect on occupancy probability, this suggests that O. briareus were equally likely to occupy each size of den unit.
Occupation behaviour of individual dens displayed subtly different results (Table 3 and Supplementary Table 1). Occupation was less likely in ‘Emmas’ than ‘Grouper Cave' (OR = 0.06, p < 0.05) and in shallower water (OR = 2.11, p < 0.01), while increasing calcareous rubble cover had no influence on occupation likelihood. However, lone dens increased the likelihood of octopus occupation compared to two-den units (OR = 11.9, p < 0.05), whereas three- and four-units displayed no significant difference in occupation likelihood. This implies non-random occupation of dens by O. briareus. Under random occupation, we would expect octopus to more regularly occupy the largest multi-den units (i.e., four-den units) as these provide the most abundant resource.
Dens occupied by octopus frequently contained items collected by the inhabiting individual (Table 4). Of the 15 occupied dens from 13 den units, seven dens contained items (47 %), with four bivalve and one crustacean species represented, and all contained at least one cleaned item. Crustacean items were only observed in a single den. Items in dens were also only observed at ‘Grouper Cave’, not at ‘Emmas’. No significant difference was seen in ecological surveys for C. elevata, P. radiata or Mytilopsis sp. abundances (Fig. 5) between experimental sites (Welch Two Sample t test: C. elevata, t(4) = 0.19, p = 0.85; P. radiata, t(4) = − 1.52, p = 0.19; Mann-Witney U test: Mytilopsis, U = 19, p = 0.16). Only two L. pictum individuals were identified in each site, whereas zero P. aculeata were observed.
The abundance of potential prey item species observed during ecological surveys at the two experimental sites ‘Grouper Cave’ and ‘Emmas’. The data are presented as a boxplot where the whiskers extend 1.5 × the interquartile range of the data and the black horizontal bar indicates the median. Outliers are indicated by a black dot. An example image of each species is also provided at the top of the figure
Discussion
Den occupation behaviour
With only two examples of adjacent occupation, zero cohabitation and no increased likelihood of occupation in den units with many dens, the use of artificial dens supports the notion that this sub-population of Octopus briareus is not social and may in fact be antisocial, not readily occupying dens that could support neighbours. This result is unexpected, as the sub-population is thought to be of high density (Aronson 1986) and other work has suggested that increasing population density has the potential to decrease aggression/increase social tolerance in fish (Ruzzante and Doyle 1993; Syarifuddin and Kramer 1996) and farm animals (Estévez et al. 2003; Estévez et al. 2007). However, the formation of dominance hierarchies (Cigliano 1993) can imply antisocial behaviour by individual animals as they avoid all or certain conspecifics.
As lone dens were more likely to be occupied than grouped dens, when considering individual den occupation rates rather than den unit rates, Sweetings Pond O. briareus appear to be antisocial (actively avoiding conspecifics as evidenced by the greater occupation of isolated dens) rather than either asocial (equal occupation of dens regardless of den unit size) or social (preferential occupation of dens with conspecifics). Cannibalism is suggested to be high in this sub-population due to the high density (Aronson 1989) so avoidance may be the best form of defence for many individuals; a plastic response in behaviour to the social conditions. Octopus are thought to display preferential den choices (Mather 1982b) so the sparse distribution of occupations despite the abundance of potential dens could result from avoidance behaviour and antisocial tendencies.
The lack of cohabitation observed here supports this supposition, particularly as examples of cohabitation are present in experimental populations of O. laqueus (Edsinger et al. 2020) and larger Pacific striped octopus (Caldwell et al. 2015), the former anecdotally described as tolerant (Edsinger et al. 2020) and, therefore, asocial by our definition. The two examples of adjacent occupancy in Sweetings Pond does, however, conflict with Aronson (1986)’s observations that no octopus occupied dens neighbouring another. Behavioural observations of naturally insular sub-populations of gloomy octopus, O. tetricus, (Scheel et al. 2016; Scheel et al. 2017 and a few deep-sea species (Graneledone sp.—Drazen et al. 2003, Muusoctopus sp. —Hartwell et al. 2018 and Vulcanoctopus hydrothermalis—Voight 2005) have established the capability of social interactions and tolerance respectively, when neighbouring individuals are consistent and in close proximity, so the lack of adjacent occupancies in Sweetings Pond, a rare, large insular marine ecosystem, is unexpected. It is possible that the area of available habitat does not aggregate animals into sufficient local density for octopus to persistently interact with the same individuals as required to establish individual recognition (D’Eath and Keeling 2003) and dominance hierarchies. This is supported by the fairly mobile nature of the species, with den occupation tenure by the majority of individuals to be <25 days in non-brooding individuals (Aronson 1989) compared to >30 days in the majority of O. bimaculatus (Ambrose 1982) or the apparently permanent, or at least long-term residency, in the model O. tetricus sub-population (Godfrey-Smith and Lawrence 2012).
Den units were randomly deployed so artefact influences of habitat/unmeasured factors are unlikely to be impactful, but the unexpectedly low occupation rates compared to those achieved in 1980s den enrichment experiments (38.3% of 60 deployments—Aronson 1986) limits the strength of any conclusions made here. This being said, occupation rates of entire den units were comparable to Aronson (1986) and PVC pots deployed in the Mediterranean targeting O. vulgaris (ranging from 18.2 to 48.5% of 105 deployments—Mereu et al. 2018). Even the low occupation rate of individual dens was similar to artificial shelters targeting the Pacific pygmy octopus, Octopus digueti, in the Gulf of California (12.1% of 6390 deployments—Voight 1992). Katsanevakis and Verriopoulos (2004) did not report the percentage of successful den occupations in their den enrichment experiment. Refuges were thought to be limiting in Sweetings Pond due to the ready occupation of artificial dens (Aronson 1986) and dominance of soft substrate (O’Brien et al. 2020, Supplementary Figure 2), yet the low occupation rate present here throws this in to doubt.
An unexpected observation of this study was that calcareous rubble (CR) cover did not influence occupation probability. This prediction conflicts with previous work on this population suggesting that octopus spatial distribution in this system is positively predicted by CR cover (O’Brien et al. 2020). Artificial dens may provide a high quality, spacious resource compared to the small natural dens typically available, and so weakens octopus’ association with CR as habitat. This is evidenced by the fact that, in a recent survey, zero octopus were observed at ‘Emmas’ (f.k.a. ‘Octopus Den'—O’Brien et al. 2020), a site that yielded five octopus individuals in this study. ‘Emmas’ is also more anthropogenically influenced than ‘Grouper Cave’ due the presence of agriculture on the lake’s edge. Ergo, artificial dens may expose the presence of rare individuals unlikely to be observed in stressed sites. From a management perspective, this result implies that artificial dens may have use as an octopus aggregating device (Katsanevakis and Verriopoulos 2004; Godfrey-Smith and Lawrence 2012) for surveying or behavioural studies if there is a limitation of den resources (i.e., on soft substrates). Katsanevakis and Verriopoulos (2004) do warn that using dens/traps for this purpose may sample a wider area than the target site due to the attraction of migrants, but in confined environments such as Sweetings Pond, abundance estimates using this technique may not be as critically impacted as in the marine environment.
Prey preference
It was unsurprising to find only five species items in artificial dens occupied by O. briareus in Sweetings Pond due to the suppressed invertebrate diversity and high abundance of these species present in the system (Aronson and Harms 1985). In the absence of prey preference data for O. briareus, other than listing of species in Aronson (1986), the number of potential prey taxa reported here are significantly less than those seen in midden studies of the trophically similar O. vulgaris, around the Caribbean island of Bonaire (n = 75—Anderson et al. 2008) and Bermuda (n = 28—Mather 1991).
Midden analysis is typically criticised for its underestimation of soft bodied prey and the dispersal of discarded hard parts by both biotic (e.g., crab removal) and abiotic (e.g., wave action) means (Ambrose 1983; Mather 1992). Consequently, feeding rates should not be estimated on an individual octopus basis (Ambrose 1983) and can only be assumed at the population level. However, in this study, because middens are generally rare in Sweetings Pond (Aronson 1989), potentially due to difficulties in discriminating middens against the calcareous rubble prevalent in many areas, and the absence of middens outside of artificial dens during this study, if blocking material versus predated items can be distinguished, artificial dens could provide a more accurate representation of prey preferences as items cannot be dispersed from inside the den. We attempted to disentangle blocking versus prey items by characterising items as cleaned or uncleaned. Applying the assumption that cleaned items are prey items, this study identifies the same bivalve and crustacean prey as listed by Aronson (1989) but also provides a frequency of occurrence for each.
Conclusion
Octopus briareus within Sweetings Pond display unbiased occupation of different den unit sizes if den unit occupation likelihood is considered in isolation, whereas isolated dens display higher likelihood of occupation than any of the other den unit sizes if considering individual den colonisation. When these predictions are combined with the rare examples of adjacent occupation, Sweetings Pond octopus appear to be less tolerant of conspecifics than expected and may even be antisocial and actively avoiding conspecifics; they are purposefully solitary. This is possibly driven by the high abundance of octopus individuals supported by the system which limits the opportunity for repeat interactions with the same individuals. The lack of habitat influence in the regression models was unexpected, with artificial dens potentially providing a novel method for octopus to inhabit otherwise non-preferable areas. No social behaviours were recorded and further research effort is required to determine whether they are absent or simply unobserved.
The majority of the examples of high octopus density, and likely social tolerance, occur in small scale environments metres to 10 s of metres in diameter. The Sweetings Pond system, therefore, may act as a confined natural environment for further experimentation to supplement to these other model systems of octopus social behaviour (particularly Godfrey-Smith and Lawrence 2012). We, therefore, encourage further usage of the system as a natural laboratory for testing hypotheses exploring the influence of density on behaviour and evolution.
Data availability
Data archiving is not mandated but data will be made available on reasonable request.
References
Ambrose RF (1982) Shelter utilization by the molluscan cephalopod Octopus bimaculatus. Marine Ecology Progress Series 7:7–73. https://doi.org/10.3354/meps007067
Ambrose RF (1983) Midden formation by octopuses: The role of biotic and abiotic factors. Marine Behaviour and Physiology 10:137–144. https://doi.org/10.1080/10236248309378613
Anderson T (1997) Habitat selection and shelter use by Octopus tetricus. Marine Ecology Progress Series 150:137–148. https://doi.org/10.3354/meps150137
Anderson RC, Wood JB, Mather JA (2008) Octopus vulgaris in the Caribbean is a specializing generalist. Mar Ecol Prog Ser 371:199–202. https://doi.org/10.3354/meps07649
Aronson RB (1986) Life-history and den ecology of Octopus briareus Robson in a marine lake. J Exp Mar Biol Ecol 95:37–56. https://doi.org/10.1016/0022-0981(86)90086-9
Aronson RB (1989) The ecology of Octopus-briareus Robson in a Bahamian saltwater lake. Am Malacol Bull 7:47–56
Aronson RB, Harms CA (1985) Ophiuroids in a Bahamian saltwater lake-the ecology of a Paleozoic-like community. Ecology 66:1472–1483. https://doi.org/10.2307/1938010
Baeza JA (2008) Protandric simultaneous hermaphroditism in the shrimps Lysmata bahiaand Lysmata intermedia. Invertebr Biol 127:181–188. https://doi.org/10.1111/j.1744-7410.2007.00122.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bintanja R, van de Wal RSW, Oerlemans J (2005) Modelled atmospheric temperatures and global sea levels over the past million years. Nature 437:125–128. https://doi.org/10.1038/nature03975
Boal JG (2006) Social recognition: a top down view of cephalopod behaviour. Vie Et Milieu 56:69–79
Caldwell RL, Ross R, Rodaniche A, Huffard CL (2015) Behavior and body patterns of the larger pacific striped octopus. PLOS One 10:e0134152. https://doi.org/10.1371/journal.pone.0134152
Cigliano J (1993) Dominance and den use in Octopus bimaculoides. Animal Behav 46:677–684. https://doi.org/10.1006/anbe.1993.1244
D’Eath RB, Keeling LJ (2003) Social discrimination and aggression by laying hens in large groups: from peck orders to social tolerance. Appl Animal Behav Sci 84:97–212. https://doi.org/10.1016/j.applanim.2003.08.010
Drazen J, Goffredi S, Schlining B, Stakes D (2003) Aggregations of egg-brooding deep-sea fish and cephalopods on the Gorda Escarpment: a reproductive hot spot. Biol Bull 205:1–7. https://doi.org/10.2307/1543439
Economakis AE, Lobel PS (1998) Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, Central Pacific Ocean. Environ Biol Fishes 51:129–139. https://doi.org/10.1023/A:1007416813214
Edsinger E, Dölen G (2018) A conserved role for serotonergic neurotransmission in mediating social behavior in octopus. Curr Biol 28:3136-3142.e3134. https://doi.org/10.1016/j.cub.2018.07.061
Edsinger E, Pnini R, Ono N, Yanagisawa R, Dever K, Miller J (2020) Social tolerance in Octopus laqueus—a maximum entropy model. PLOS One 15:e0233834. https://doi.org/10.1371/journal.pone.0233834
Emery NJ, Clayton NS (2004) The mentality of crows: Convergent evolution of intelligence in corvids and apes. Science 306:1903–1907. https://doi.org/10.1126/science.1098410
Estévez I, Keeling LJ, Newberry RC (2003) Decreasing aggression with increasing group size in young domestic fowl. Appl Anim Behav Sci 84:213–218. https://doi.org/10.1016/j.applanim.2003.08.006
Estévez I, Andersen I, Nævdal E (2007) Group size, density and social dynamics in farm animals. Appl Anim Behav Sci 103:185–204. https://doi.org/10.1016/j.applanim.2006.05.025
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D’Angelo L, Dickel L, Gestal C, Grasso F, Kuba M, Mark F, Melillo D, Osorio D, Perkins K, Ponte G, Shashar N, Smith D, Smith J, Andrews PL (2015) Guidelines for the care and welfare of Cephalopods in research—a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab Anim 49:1–90. https://doi.org/10.1177/0023677215580006
Fleming K, Johnston P, Zwartz D, Yokoyama Y, Lambeck K, Chappell J (1998) Refining the eustatic sea-level curve since the last Glacial maximum using far- and intermediate-field sites. Earth Planet Sci Lett 163:327–342. https://doi.org/10.1016/S0012-821X(98)00198-8
Forsythe J, Hanlon R (1997) Foraging and associated behavior by Octopus cyanea Gray, 1849 on a coral atoll, French Polynesia. J Exp Mar Biol Ecol 209:15–31. https://doi.org/10.1016/S0022-0981(96)00057-3
Garci M, Hernández-Urcera J, Gilcoto M, Fernández-Gago R, González Á, Guerra Á (2016) From brooding to hatching: New insights from a female Octopus vulgaris in the wild. J Mar Biol Assoc 96:1341–1346. https://doi.org/10.1017/S0025315415001800
Gisburne TJ, Connor RC (2015) Group size and feeding success in strand-feeding bottlenose dolphins (Tursiops truncatus) in Bull Creek, South Carolina. Mar Mam Sci 31:1252–1257. https://doi.org/10.1111/mms.12207
Godfrey-Smith P, Lawrence M (2012) Long-term high-density occupation of a site by Octopus tetricus and possible site modification due to foraging behavior. Mar Freshwater Behav Physiol 45:1–8. https://doi.org/10.1080/10236244.2012.727617
Guerra A (1981) Spatial distribution pattern of Octopus vulgaris. J Zool 195:133–146. https://doi.org/10.1111/j.1469-7998.1981.tb01897.x
Guerra A, Hernandez-Urcera J, Garci ME, Sestelo M, Regueira M, Gonzalez AF, Cabanellas-Reboredo M, Calvo-Manazza M, Morales-Nin B (2014) Dwellers in dens on sandy bottoms: ecological and behavioural traits of Octopus vulgaris. Sci Mar 78:405–414. https://doi.org/10.3989/scimar.04071.28F
Hamilton WD (1964) Genetical evolution of social behaviour I. J Theor Biol 7:1–16. https://doi.org/10.1016/0022-5193(64)90038-4
Hanlon RT (1977) Laboratory rearing of the Atlantic reef octopus, Octopus briareus Robson, and its potential for mariculture. World Aquacult Soc 8:471–482. https://doi.org/10.1111/j.1749-7345.1977.tb00137.x
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.3.3.0.
Hartwell AM, Voight JR, Wheat CG (2018) Clusters of deep-sea egg-brooding octopods associated with warm fluid discharge: an ill-fated fragment of a larger, discrete population? Deep Sea Res Part I Oceanograph Res Paper 135:1–8. https://doi.org/10.1016/j.dsr.2018.03.011
Hartwick EB, Ambrose RF, Robinson SMC (1984) Den utilization and the movements of tagged Octopus dofleini. Mar Behav Physiol 11:95–110. https://doi.org/10.1080/10236248409387038
Huffard C, Caldwell R, Boneka F (2008) Mating behavior of Abdopus aculeatus (d’Orbigny 1834) (Cephalopoda: Octopodidae) in the wild. Mar Biol 154:353–362. https://doi.org/10.1007/s00227-008-0930-2
Huffard C, Caldwell R, Boneka F (2010) Male-male and male-female aggression may influence mating associations in wild Octopuses (Abdopus aculeatus). J Comp Psychol 124:38–46. https://doi.org/10.1037/a0017230
Ibanez CM, Keyl F (2010) Cannibalism in cephalopods. Rev Fish Biol Fisheries 20:123–136. https://doi.org/10.1007/s11160-009-9129-y
Jereb P, Roper CFE, Norman MD, Finn JK (eds) (2014) Cephalopods of the world, an annotated and illustrated catalogue of cephalopod species known to date, 4th edn. FAO, Rome
Katsanevakis S, Verriopoulos G (2004) Den ecology of Octopus vulgaris Cuvier, 1797, on soft sediment: availability and types of shelter. Sci Mar 68:147–5. https://doi.org/10.3989/scimar.2004.68n1147
Kayes RJ (1974) The daily activity pattern of Octopus vulgaris in a natural habitat. Mar Behav Physiol 2:337–343. https://doi.org/10.1080/10236247309386935
Legac M (1969) Some observations on the building up of hiding places by the Octopus vulgaris. Lam. in the channel region of the North-eastern Adriatic. Thalassia Jugoslavica 5:193–198
Magurran A (1990) The adaptive significance of schooling as an anti-predator defence in fish. Annales Zoologici Fennici 27:51–66
Masonjones H, Rose E, Elson J, Roberts B, Curtis-Quick J (2019) High density, early maturing, and morphometrically unique Hippocampus erectus population makes a Bahamian pond a priority site for conservation. Endang Species Res 39:35–49. https://doi.org/10.3354/esr00949
Mather JA (1980) Social organization and use of space by Octopus joubini in a semi-natural situation. Bull Mar Sci 30:848–857. https://doi.org/10.1177/0539018418785485
Mather JA (1982) Factors affecting the spatial distribution of natural populations of Octopus joubini robson. Anim Behav 30:1166–1170. https://doi.org/10.1016/S0003-3472(82)80207-8
Mather JA (1982) Choice and competition: their effects on occupancy of shell homes by Octopus joubini. Mar Behav Physiol 8:285–293. https://doi.org/10.1080/10236248209387025
Mather JA (1985) Behavioural interactions and activity of captive Eledone moschata: laboratory investigations of a ‘social’ octopus. Anim Behav 33:1138–1144. https://doi.org/10.1016/S0003-3472(85)80173-1
Mather JA (1988) Daytime activity of juvenile Octopus vulgaris in Bermuda. Malacologia 29:69–76
Mather JA (1991) Foraging, feeding and prey remains in middens of juvenile Octopus vulgaris (Mollusca: Cephalopoda). J Zool 224:27–39. https://doi.org/10.1111/j.1469-7998.1991.tb04786.x
Mather JA (1992) Interactions of juvenile Octopus vulgaris with scavaging and territorial fishes. Mar Behav Physiol 19:175–182. https://doi.org/10.1080/10236249209378806
Mather JA, Dickel L (2017) Cephalopod complex cognition. Curr Opin Behav Sci 16:131–137. https://doi.org/10.1016/j.cobeha.2017.06.008
Mather JA, Scheel D (2014) Behaviour. In: Iglesias J, Fuentes L, Villanueva R (eds) Cephalopod culture, 1st edn. Springer, Netherlands, pp 17–39. https://doi.org/10.1007/978-94-017-8648-5_2
Mereu M, Cau A, Agus B, Cannas R, Follesa MC, Pesci P, Cuccu D (2018) Artificial dens as a management tool for Octopus vulgaris: evidence from a Collaborative Fisheries Research project (central western Mediterranean Sea). Ocean Coastal Manag 165:428–433. https://doi.org/10.1016/j.ocecoaman.2018.09.006
Morse P, Zenger KR, McCormick MI, Meekan MG, Huffard CL (2017) Chemical cues correlate with agonistic behaviour and female mate choice in the southern blue-ringed octopus, Hapalochlaena maculosa (Hoyle, 1883) (Cephalopoda: Octopodidae). J Mollus Stud 83:79–87. https://doi.org/10.1093/mollus/eyw045
O’Brien DA, Taylor ML, Masonjones HD, Boersch-Supan PH, O’Shea OR (2020) Drivers of octopus abundance and density in an anchialine lake: a 30 year comparison. J Exp Mar Biol Ecol 528:151377. https://doi.org/10.1016/j.jembe.2020.151377
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2019) Vegan: community Ecology Package, R package version 2.5-6. URL: https://CRAN.R-project.org/package=vegan
Oliveira RF (2012) Social plasticity in fish: integrating mechanisms and function. J Fish Biol 81:2127–2150. https://doi.org/10.1111/j.1095-8649.2012.03477.x
Peterson CH (1983) Interactions between two infaunal bivalves, Chione undatella (Sowerby) and Protothaca staminea (Conrad), and two potential enemies, Crepidula onyx (Sowerby) and Cancer anthonyi (Rathbun). J Exp Mar Biol Ecol 68:145–158. https://doi.org/10.1016/0022-0981(83)90156-9
QGIS Development Team, (2019). QGIS Geographic Information System. Open source geospatial foundation project. URL: http://qgis.osgeo.org.
R Core Team, 2018. R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. URL: https://www.R-project.org/.
Ritz DA, Hobday AJ, Montgomery JC, Ward AJ (2011) Social aggregation in the pelagic zone with special reference to fish and invertebrates. Adv Mar Biol 60:161–227. https://doi.org/10.1016/B978-0-12-385529-9.00004-4
Roper C, Sweeney J, Nauen C (1984) Cephalopods of the world, an annotated and illustrated catalogue of species of interest to fisheries. FAO Fish Synop 3:1–127
Rubenstein D, Abbot P (2017) The evolution of social evolution. In: Rubenstein D, Abbot P (eds) Comparative social evolution. Cambridge University Press, Cambridge, pp 1–18
Rubenstein DI, Rubenstein DR (2013). Social behavior. In: Levin SA (ed.) Encyclopedia of biodiversity (2nd edn), Academic Press, p 571-579. https://doi.org/10.1016/B978-0-12-384719-5.00126-X.
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Image J2: ImageJ for the next generation of scientific image data. Bmc Bioinform 18:529. https://doi.org/10.1186/s12859-017-1934-z
Ruiz-Gomez M, Kittilsen S, Hoglund E, Huntingford FA, Sorensen C, Pottinger T, Bakken M, Winberg S, Korzan W, Overli O (2008) Behavioral plasticity in rainbow trout (Oncorhynchus mykiss) with divergent coping styles: when doves become hawks. Horm Behavior 54:534–538. https://doi.org/10.1016/j.yhbeh.2008.05.005
Ruzzante D, Doyle R (1993) Evolution of social behavior in a resource-rich, structured environment: Selection experiments with medaka (Oryzias latipes). Evolution 47:456–470. https://doi.org/10.2307/2410064
Scheel D, Bisson L (2012) Movement patterns of giant Pacific octopuses, Enteroctopus dofleini (Wülker, 1910). J Exp Mar Biol Ecol 416–417:21–31. https://doi.org/10.1016/j.jembe.2012.02.004
Scheel D, Godfrey-Smith P, Lawrence M (2016) Signal use by octopuses in agonistic interactions. Curr Biol 26:377–382. https://doi.org/10.1016/j.cub.2015.12.033
Scheel D, Chancellor S, Hing M, Lawrence M, Linquist S, Godfrey-Smith P (2017) A second site occupied by Octopus tetricus at high densities, with notes on their ecology and behavior. Mar Freshwater Behav Physiol 50:285–291. https://doi.org/10.1080/10236244.2017.1369851
Simpson EH (1949) Measurement of diversity. Nature 163:688–688. https://doi.org/10.1038/163688a0
Subramoniam T (2013) Origin and occurrence of sexual and mating systems in Crustacea: a progression towards communal living and eusociality. J Biosci 38:951–969. https://doi.org/10.1007/s12038-013-9392-x
Syarifuddin S, Kramer DL (1996) The effect of group size on space use and aggression at a concentrated food source in blue gouramis, Trichogaster trichopterus (Pisces: Belontiidae). Environ Biol Fish 46:289–296. https://doi.org/10.1007/BF00005005
Szumilas M (2010) Explaining odds ratios. J Can Acad Child Adolesc Psych 19:227–229
Tricarico E, Borrelli L, Gherardi F, Fiorito G (2011) I know my neighbour: individual recognition in Octopus vulgaris. PLOS One 6:e18710. https://doi.org/10.1371/journal.pone.0018710
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York. https://doi.org/10.1007/978-0-387-21706-2
Vidal EA, Villanueva R, Andrade JP, Andrade JP, Gleadall IG, Iglesias J, Koueta N, Rosas C, Segawa S, Grasse B, Franco-Santos RM, Albertin CB, Caamal-Monsreal C, Chimal ME, Edsinger-Gonzales E, Gallardo P, Le Pabic C, Pascual C, Roumbedakis K, Wood J (2014) Cephalopod culture: current status of main biological models and research priorities. Adv Mar Biol 67:1–98. https://doi.org/10.1016/b978-0-12-800287-2.00001-9
Voight JR (1992) Movement, injuries and growth of members of a natural population of the Pacific pygmy octopus, Octopus digueti. J Zool 228:247–264. https://doi.org/10.1111/j.1469-7998.1992.tb04606.x
Voight JR (2005) Hydrothermal vent octopuses of Vulcanoctopus hydrothermalis, feed on bathypelagic amphipods of Halice hesmonectes. J Mar Biol Assoc 85:985–988. https://doi.org/10.1017/s0025315405011999
Walderon MD, Nolt KJ, Haas RE, Prosser KN, Holm JB, Nagle GT, Boal JG (2011) Distance chemoreception and the detection of conspecifics in Octopus bimaculoides. J Mollus Stud 77:309–311. https://doi.org/10.1093/mollus/eyr009
Zwarts L, Wanink J (1989) Siphon size and burying depth in deposit and suspension-feeding benthic bivalves. Mar Biol 100:227–240. https://doi.org/10.1007/BF00391963
Acknowledgements
The authors would like to thank Will Burchell and Enrique Bethel for their assistance in the field. We also thank the reviewers for insightful and constructive feedback on earlier versions of this manuscript which improved the quality of work.
Funding
This work was supported by grants awarded to O.O by the Lyford Cay Foundation (grant number LCF8008) the British Ecological Society (grant number OR19/113), and a grant awarded to H.M. from The University of Tampa.
Author information
Authors and Affiliations
Contributions
Conceptualization: D. A. O’Brien, H. D. Masonjones and O. R. O’Shea; Methodology: D. A. O’Brien; Formal analysis and investigation: D. A. O’Brien and P. H Boersch-Supan; Writing-original draft preparation: D. A. O’Brien; Writing-review and editing: M. L. Taylor, H. D. Masonjones, P. H Boersch-Supan and O. R. O’Shea; Funding acquisition: O. R. O’Shea; Resources: H. D. Masonjones and O. R. O’Shea Supervision: M. L. Taylor and O. R. O’Shea.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research was carried out on a protected animal and was, therefore, regulated by the Animals (Scientific Procedures) Act 1986 and Directive 2010/63/EU. After review by the University of Essex Animal Welfare and Ethical Review Panel (UK), the research was considered to be following UK Home Office standards. A copy of the review is available upon request. This study was approved by The Bahamian Government’s Department of Marine Resources, permit reference: MAMRFIS9.
Consent for publication
All individuals listed as authors agreed to be listed and approve the submitted version of the manuscript.
Additional information
Reviewers: D. Scheel and an undisclosed expert.
Responsible Editor: H.-J. Hoving.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Brien, D.A., Taylor, M.L., Masonjones, H.D. et al. An experimental assessment of social tolerance and den ecology in a high-density octopus population. Mar Biol 168, 61 (2021). https://doi.org/10.1007/s00227-021-03865-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03865-4