Abstract
Purpose
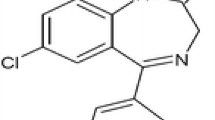
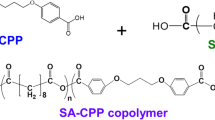
Risperidone is an important antipsychotic agent used for treatment of both acute and chronic schizophrenia. The drug has been effective in resolving the disease’s signs while keeping extrapyramidal side effects as low as possible. To achieve a sustained and gradual release of risperidone from PLGA microspheres, we utilized an emulsifying agent; PLGA-PEG-PLGA triblock (M-triblock), instead of PVA (M-PVA), applying ring-opening polymerization of the triblock via supercritical carbon dioxide (scCO2).
Methods
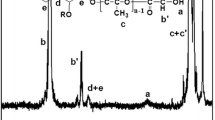
PVA and PLGA-PEG-PLGA (as emulsifiers) were successfully employed to prepare risperidone-loaded PLGA microspheres. The scCO2 and microwave irradiation methods were used to construct PLGA-PEG-PLGA copolymers. The microspheres were subjected to various analyses to determine the particles’ sizes, morphological characteristics, and structure (via XRD) and in vitro drug release kinetics. Drug loading capacity and encapsulation percentage were also determined.
Results
The results showed a mean diameter of 43.34 ± 2.30 µm for M-PVA, and that of the M-triblock was 57.49 ± 1.21 µm. Risperidone encapsulation percentages were 72.8 ± 2.09 for M-PVAs and 73.4 ± 2.41 for the M-triblock. Particle size, risperidone distribution, and drug loading and encapsulation percentages were similar between M-PVAs and the M-triblock. In scanning electron microscopy (SEM), the morphology of the triblock emulsifier was more uniform than PVAs.
Conclusions
The triblock emulsifier showed slower initial drug release than PVAs due to the precipitation of the triblock hydrogel around PLGA microspheres. Overall, the triblock used here can be a good alternative to PVA.










Similar content being viewed by others
Abbreviations
- PEG:
-
Polyethylene glycol
- PLGA:
-
Poly lactic-co-glycolic acid
- Triblock:
-
PLGA-PEG-PLGA
- M-triblock:
-
Microsphere via PLGA-PEG-PLGA as an emulsifier
- PVA:
-
Poly(vinyl alcohol)
- M-PVA:
-
Microsphere via PVA as an emulsifier
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
- DCM:
-
Dichloromethane
- FDA:
-
U.S. Food and Drug Administration
- GA:
-
Glycolide
- LA:
-
D,L-lactide
- FID:
-
Flame ionization detector
- GPC:
-
Gel permeation chromatography
- 1H-NMR:
-
Proton-nuclear magnetic resonance
- PDI:
-
Polydispersity index
- SEC:
-
Size exclusion chromatography
- A s/A is :
-
Areasample/Ainternal standard
- PBS:
-
Phosphate buffer solution
- THF:
-
Tetrahydrofuran
References
Hori H, Katsuki A, Atake K, Yoshimura R. Effects of Continuing Oral Risperidone vs. Switching from Risperidone to Risperidone Long-Acting Injection on Cognitive Function in Stable Schizophrenia Patients: A Pilot Study. Front Psych 2018;9(74). https://doi.org/10.3389/fpsyt.2018.00074
Covell NH, McEvoy JP, Schooler NR, Stroup TS, Jackson C, Rojas I, Essock SM, Network ST. Effectiveness of switching from long-acting injectable fluphenazine or haloperidol decanoate to long-acting injectable risperidone microspheres. J Clin Psychiatry. 2012;73(5):669.
Shen J, Choi S, Qu W, Wang Y, Burgess DJ. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J Control Release. 2015;218:2–12.
Wilson C, Washington N, Peach J, Murray G, Kennerley J. The behaviour of a fast-dissolving dosage form (Expidet) followed by γ-scintigraphy. Int J Pharm. 1987;40(1–2):119–23.
Song J, Xie J, Li C, Lu JH, Meng QF, Yang Z, Lee RJ, Wang D, Teng LS. Near infrared spectroscopic (NIRS) analysis of drug-loading rate and particle size of risperidone microspheres by improved chemometric model. Int J Pharm. 2014;472(1–2):296–303.
Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, Davis JM. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. The Lancet. 2012;379(9831):2063–71.
Harrison TS, Goa KL. Long-acting risperidone CNS Drugs. 2004;18(2):113–32.
Abu-Thabit NY, Makhlouf ASH. Historical development of drug delivery systems: From conventional macroscale to controlled, targeted, and responsive nanoscale systems. In: Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, 2018;1:3–41. Elsevier.
D’Souza S, Faraj JA, Giovagnoli S, DeLuca PP. Development of risperidone PLGA microspheres. Journal of Drug Delivery 2014.
Blader JC, Pliszka SR, Kafantaris V, Foley CA, Carlson GA, Crowell JA, Bailey BY, Sauder C, Daviss WB, Sinha C. Stepped treatment for attention-deficit/hyperactivity disorder and aggressive behavior: a randomized, controlled trial of adjunctive risperidone, divalproex sodium, or placebo after stimulant medication optimization. J Am Acad Child Adolesc Psychiatry 2020.
Su Z, Sun F, Shi Y, Jiang C, Meng Q, Teng L, Li Y. Effects of formulation parameters on encapsulation efficiency and release behavior of risperidone poly (D, L-lactide-co-glycolide) microsphere. Chem Pharm Bull. 2009;57(11):1251–6.
Oroojalian F, Jahanafrooz Z, Chogan F, Rezayan AH, Malekzade E, Rezaei SJT, Nabid MR, Sahebkar A. Synthesis and evaluation of injectable thermosensitive penta-block copolymer hydrogel (PNIPAAm-PCL-PEG-PCL-PNIPAAm) and star-shaped poly (CL─ CO─ LA)-b-PEG for wound healing applications. J Cell Biochem. 2019;120(10):17194–207.
Selmin F, Blasi P, DeLuca PP. Accelerated polymer biodegradation of risperidone poly (D, L-lactide-co-glycolide) microspheres. AAPS PharmSciTech. 2012;13(4):1465–72.
Yerragunta B, Jogala S, Chinnala KM, Aukunuru J. Development of a novel 3-month drug releasing risperidone microspheres. J Pharm Bioallied Sci. 2015;7(1):37.
Di Francesco M, Primavera R, Summa M, Pannuzzo M, Di Francesco V, Di Mascolo D, Bertorelli R, Decuzzi P. Engineering shape-defined PLGA microPlates for the sustained release of anti-inflammatory molecules. J Control Release. 2020;319:201–12.
Hu B, Yan H, Sun Y, Chen X, Sun Y, Li S, Jing Y, Li H. Organogels based on amino acid derivatives and their optimization for drug release using response surface methodology. Artificial Cells, Nanomedicine, and Biotechnology. 2020;48(1):266–75.
Park EJ, Amatya S, Kim MS, Park JH, Seol E, Lee H, Shin Y-H, Na DH. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch Pharmacal Res. 2013;36(6):651–9.
D’Souza S, Faraj J, DeLuca P. Microsphere delivery of Risperidone as an alternative to combination therapy. Eur J Pharm Biopharm. 2013;85(3):631–9.
Beauchemin M, Geguchadze R, Guntur AR, Nevola K, Le PT, Barlow D, Rue M, Vary CP, Lary CW, Motyl KJ. Exploring mechanisms of increased cardiovascular disease risk with antipsychotic medications: risperidone alters the cardiac proteomic signature in mice. Pharmacol Res. 2020;152:104589.
Su Z-X, Shi Y-N, Teng L-S, Li X, Wang L-X, Meng Q-F, Teng L-R, Li Y-X. Biodegradable poly (D, L-lactide-co-glycolide)(PLGA) microspheres for sustained release of risperidone: zero-order release formulation. Pharm Dev Technol. 2011;16(4):377–84.
Ereshefsky L, Mannaert E. Pharmacokinetic profile and clinical efficacy of long-acting risperidone. Drugs in R & D. 2005;6(3):129–37.
Piacentino D, Kotzalidis GD, Schoretsanitis G, Paulzen M, Haen E, Cappelletti S, Giupponi G, Grözinger M, Conca A. Plasma risperidone-related measures in children and adolescents with oppositional defiant/conduct disorders. Clinical Psychopharmacology and Neuroscience. 2020;18(1):41.
Elstad NL, Fowers KD. OncoGel (ReGel/paclitaxel)—clinical applications for a novel paclitaxel delivery system. Adv Drug Deliv Rev. 2009;61(10):785–94.
Daneshvar M, Kamali H, Masoomi M, Ghaziaskar H. Supercritical carbon dioxide grafting of glycidyl methacrylate onto medium density polyethylene and purification of residual monomer and initiator. J Supercrit Fluids. 2012;70:119–25.
Jalilvand M, Kamali H, Nematollahi A. Pressurized fluid extraction of rice bran oil using a modified supercritical fluid extractor and a central composite design for optimization. J Liq Chromatogr Relat Technol. 2013;36(11):1562–74.
Watanabe M, Hashimoto Y, Kimura T, Kishida A. Characterization of engineering plastics plasticized using supercritical CO2. Polymers. 2020;12(1):134.
Moghadas BK, Akbarzadeh A, Azadi M, Aghili A, Rad AS, Hallajian S, The morphological properties and biocompatibility studies of synthesized nanocomposite foam from modified polyethersulfone/graphene oxide using supercritical CO2. J Macromol Sci A. 2020;1–10.
Jeong B, Bae YH, Kim SW. Biodegradable thermosensitive micelles of PEG-PLGA-PEG triblock copolymers. Colloids Surf, B. 1999;16(1):185–93.
Khodaverdi E, Akbari A, Tekie FSM, Mohajeri SA, Zohuri G, Hadizadeh F. Sustained delivery of amphotericin B and vancomycin hydrochloride by an injectable thermogelling tri-block copolymer. PDA J Pharm Sci Technol 2013;67.
Khodaverdi E, Tekie FSM, Mohajeri SA, Ganji F, Zohuri G, Hadizadeh F. Preparation and investigation of sustained drug delivery systems using an injectable, thermosensitive, in situ forming hydrogel composed of PLGA–PEG–PLGA. AAPS PharmSciTech. 2012;13(2):590–600.
Kamali H, Khodaverdi E, Hadizadeh F. Ring-opening polymerization of PLGA-PEG-PLGA triblock copolymer in supercritical carbon dioxide. J Supercrit Fluids. 2018;137:9–15.
Zhan S, Wan Z, Zhao Y, Wang J, Li Z. Ring-opening dispersion polymerization of L-lactide initiated by L-arginine in supercritical carbon dioxide. Polym Degrad Stab. 2020;171:109049.
Zentner GM, Rathi R, Shih C, McRea JC, Seo M-H, Oh H, Rhee B, Mestecky J, Moldoveanu Z, Morgan M. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72(1):203–15.
Alami-Milani M, Zakeri-Milani P, Valizadeh H, Fathi M, Salatin S, Salehi R, Jelvehgari M. PLA-PCL-PEG-PCL-PLA based micelles for improving the ocular permeability of dexamethasone: development, characterization, and in vitro evaluation. Pharm Dev Technol (just-accepted). 2020;1–48.
Choonara YE, Kondiah PPD, Kondiah PJ, Kumar P, Du Toit LC, Marimuthu T, Pillay VA. Thermoresponsive Hydrogel. In. US Patent App. 2020;16/482,166.
Liu W, Jin X, Yao S, Wang F. Determination of risperidone and 9-Hydroxyrisperidone in human serum by heart-cutting isocratic two-dimensional liquid chromatography. Anal Lett. 2020;1–18.
Schaefer M, Sarkar S, Theophil I, Leopold K, Heinz A, Gallinat J. Acute and long-term memantine add-on treatment to risperidone improves cognitive dysfunction in patients with acute and chronic schizophrenia. Pharmacopsychiatry. 2020;53(01):21–9.
Iwata N, Ishigooka J, Naoi I, Matsumoto M, Kanamori Y, Nakamura H, Higuchi T. Long-term safety and efficacy of blonanserin transdermal patches in Japanese patients with schizophrenia: a 52-week open-label. Multicenter Study CNS drugs. 2020;34(1):103–16.
Rahimizadeh M, Eshghi H, Shiri A, Ghadamyari Z, Matin MM, Oroojalian F, Pordeli P. Fe (HSO 4) 3 as an efficient catalyst for diazotization and diazo coupling reactions. J Korean Chem Soc. 2012;56(6):716–9.
Rawat A, Bhardwaj U, Burgess DJ. Comparison of in vitro–in vivo release of Risperdal® Consta® microspheres. Int J Pharm. 2012;434(1–2):115–21.
Rawat A, Stippler E, Shah VP, Burgess DJ. Validation of USP apparatus 4 method for microsphere in vitro release testing using Risperdal® Consta®. Int J Pharm. 2011;420(2):198–205.
An T, Choi J, Kim A, Lee JH, Nam Y, Park J, Kyung Sun B, Suh H, Kim CJ, Hwang SJ. Sustained release of risperidone from biodegradable microspheres prepared by in-situ suspension-evaporation process. Int J Pharm 2020;503(1–2), 8–15.
Lin X, Xu Y, Tang X, Zhang Y, Chen J, Zhang Y, He H, Yang Z. A uniform ultra-small microsphere/SAIB hybrid depot with low burst release for long-term continuous drug release. Pharm Res. 2015;32(11):3708–21.
Mosafer J, Abnous K, Tafaghodi M, Mokhtarzadeh A, Ramezani M. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur J Pharm Biopharm. 2017;113:60–74.
Hu Z, Liu Y, Yuan W, Wu F, Su J, Jin T. Effect of bases with different solubility on the release behavior of risperidone loaded PLGA microspheres. Colloids Surf, B. 2011;86(1):206–11.
Mazzoli A, Favoni O. Particle size, size distribution and morphological evaluation of airborne dust particles of diverse woods by scanning electron microscopy and image processing program. Powder Technol. 2012;225:65–71.
Andhariya JV, Shen J, Choi S, Wang Y, Zou Y, Burgess DJ. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J Control Release. 2017;255:27–35.
Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa-McMillan A. Maintenance treatment with long-acting injectable risperidone in first-episode schizophrenia: a randomized effectiveness study. J Clin Psychiatry. 2012;73(9):1224–33.
Mouez MA, Zaki NM, Mansour S, Geneidi AS. Bioavailability enhancement of verapamil HCl via intranasal chitosan microspheres. Eur J Pharm Sci. 2014;51:59–66.
He J, Wang W, Long F, Zou Z, Fu Z, Xu Z. Hydrothermal synthesis of hierarchical rose-like Bi2WO6 microspheres with high photocatalytic activities under visible-light irradiation. Mater Sci Eng, B. 2012;177(12):967–74.
Wang S, Wang L, Yang T, Liu X, Zhang J, Zhu B, Zhang S, Huang W, Wu S. Porous α-Fe2O3 hollow microspheres and their application for acetone sensor. J Solid State Chem. 2010;183(12):2869–76.
Rashidi A, Omidi M, Choolaei M, Nazarzadeh M, Yadegari A, Haghierosadat F, Oroojalian F, Azhdari M. Electromechanical properties of vertically aligned carbon nanotube. In: Advanced Materials Research 2013;332–336. Trans Tech Publ
Desai K-GH, Schwendeman SP. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J Control Release. 2013;165(1):62–74.
Della Porta G, Adami R, Del Gaudio P, Prota L, Aquino R, Reverchon E. Albumin/gentamicin microspheres produced by supercritical assisted atomization: optimization of size, drug loading and release. J Pharm Sci. 2010;99(11):4720–9.
Deshmukh RK, Naik JB. Diclofenac sodium-loaded Eudragit® microspheres: optimization using statistical experimental design. J Pharm Innov. 2013;8(4):276–87.
Sojitra C, Tehare A, Dholakia C, Sudhakar P, Agarwal S, Singh KK. Development and validation of residual solvent determination by headspace gas chromatography in Imatinib Mesylate API SN. Appl Sci. 2019;1(3):233.
Andhariya JV, Choi S, Wang Y, Zou Y, Burgess DJ, Shen J. Accelerated in vitro release testing method for naltrexone loaded PLGA microspheres. Int J Pharm. 2017;520(1–2):79–85.
Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31(14):4113–20.
Castor TP. Polymer microspheres/nanospheres and encapsulating therapeutic proteins therein. In. Google Patents 2013.
Khodaverdi E, Honarmandi R, Alibolandi M, Baygi RR, Hadizadeh F, Zohuri G. Evaluation of synthetic zeolites as oral delivery vehicle for anti-inflammatory drugs. Iran J Basic Med Sci. 2014;17(5):337.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71.
Riley T, Christopher D, Arp J, Casazza A, Colombani A, Cooper A, Dey M, Maas J, Mitchell J, Reiners M. Challenges with developing in vitro dissolution tests for orally inhaled products (OIPs). AAPS PharmSciTech. 2012;13(3):978–89.
Ige PP, Baria RK, Gattani SG. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf, B. 2013;108:366–73.
Jahromi LP, Ghazali M, Ashrafi H, Azadi A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon. 2020;6(2):e03451.
Gomes-Filho MS, Barbosa MAA, Oliveira FA. A statistical mechanical model for drug release: relations between release parameters and porosity. Phys A. 2020;540:123165.
Khodaverdi E, Hadizadeh F, Tekie FSM, Jalali A, Mohajeri SA, Ganji F. Preparation and analysis of a sustained drug delivery system by PLGA–PEG–PLGA triblock copolymers. Polym Bull. 2012;69(4):429–38.
Hameed HA, Khan S, Shahid M, Ullah R, Bari A, Ali SS, Hussain Z, Sohail M, Khan SU, Htar TT. Engineering of naproxen loaded polymer hybrid enteric microspheres for modified release tablets: development, characterization, in silico modelling and in vivo evaluation. Drug Des Dev Ther. 2020;14:27.
Yang Z, Cai W. Surfactant-free preparation of mesoporous solid/hollow boehmite and bayerite microspheres via double hydrolysis of NaAlO2 and formamide from room temperature to 180° C. J Colloid Interface Sci. 2020;564:182–92.
Luan X, Skupin M, Siepmann J, Bodmeier R. Key parameters affecting the initial release (burst) and encapsulation efficiency of peptide-containing poly (lactide-co-glycolide) microparticles. Int J Pharm. 2006;324(2):168–75.
Yang Z, Liu L, Su L, Wu X, Wang Y, Liu L, Lin X. Design of a zero-order sustained release PLGA microspheres for palonosetron hydrochloride with high encapsulation efficiency. Int J Pharm. 2020;575:119006.
Khodaverdi E, Tafaghodi M, Ganji F, Abnoos K, Naghizadeh H. In vitro insulin release from thermosensitive chitosan hydrogel. AAPS PharmSciTech. 2012;13(2):460–6.
Khodaverdi E, Farhadi F, Jalali A, Mirzazadeh Tekie FS. Preparation and investigation of poly (N-isopropylacrylamide-acrylamide) membranes in temperature responsive drug delivery. Iran J Basic Med Sci. 2010;13(3):102–10.
Singh J, Gupta S, Kaur H. Prediction of in vitro drug release mechanisms from extended release matrix tablets using SSR/R2 technique. Trends Applied Sci Res. 2011;6:400–9.
Khodaverdi E, Rajabi O, Abdekhodai M, X Yu Wu. A novel composite membrane for PH responsive permeation. Iran J Basic Med Sci. 2008;11(2):70–9.
Chime SA, Attama AA, Builders PF, Onunkwo GC. Sustained-release diclofenac potassium-loaded solid lipid microparticle based on solidified reverse micellar solution: in vitro and in vivo evaluation. J Microencapsul. 2013;30(4):335–45.
Atyabi F, Khodaverdi E, Dinarvand R. Temperature modulated drug permeation through liquid crystal embedded cellulose membranes. Int J Pharm. 2007;339(1–2):213–21.
Rahimi M, Mobedi H, Behnamghader A. In situ-forming PLGA implants loaded with leuprolide acetate/β-cyclodextrin complexes: mathematical modelling and degradation. J Microencapsul. 2016;33(4):355–64.
Funding
This work was supported by the grant number 951793 from Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadpour, F., Kamali, H., Hadizadeh, F. et al. The PLGA Microspheres Synthesized by a Thermosensitive Hydrogel Emulsifier for Sustained Release of Risperidone. J Pharm Innov 17, 712–724 (2022). https://doi.org/10.1007/s12247-021-09544-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09544-7