Abstract
4-chlorophenol (4-CP) is recognized as a highly toxic organic which can cause damage effects on human life and the aquatic environment. Since conventional wastewater techniques were unable to remove non-biodegradable chlorophenols, advanced oxidation process was investigated to achieve this goal. Amongst them, non-thermal plasma was recently proposed as an alternative and promising technique. However, the energy efficiency of most non-thermal plasma technique is very low as well as the input energy is dissipated in many forms such as heat, UV radiation, electromagnetic waves, etc., that are not totally involved in the pollutant degradation. In this work, quantitative analyses of UV radiation and H2O2 in a moist air gliding arc plasma (glidarc) were performed using potassium ferrioxalate actinometry and peroxytitanyl complex method, respectively. The role of UV light emitted in gliding arc plasma was elucidated by performing parallel experiments: H2O2, glidarc alone, glidarc + H2O2, glidarc + H2O2 + Fe3+ (plasma-Photo-Fenton). The results show that the incorporation of Fenton reagents in 4-CP solution exposed to the plasma enhanced the yields of chemical active species, which were available for efficient removal and mineralization of 4-CP.
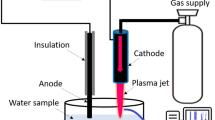
Graphic Abstract









Similar content being viewed by others
References
Zhou T, Li Y, Ji J, Wong FS, Lu X (2008) Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: kinetic, pathway and effect factors. Sep Purif Technol 62:551–558
Wang M, Fang G, Liu P, Zhou D, Ma C, Zhang D, Zhan J (2016) Fe3O4@β-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl Catal B Environ 188:113–122
Kennedy LJ, Vijaya JJ, Kayalvizhi K, Sekaran G (2007) Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chem Eng J 132:279–287
Keith LH, Telliard WA (1979) Priority pollutants. I. A perspective view. Environ Sci Technol 13:416–423
Johnson I, Atkinson C, Hope S-J, Sorokin N (2007) Proposed EQS for Water Framework Directive Annex VIII substances: 2,4-dichlorophenol.
Tarkwa JB, Oturan N, Acayanka E, Laminsi S, Oturan MA (2019) Photo-Fenton oxidation of Orange G azo dye: process optimization and mineralization mechanism. Environ Chem Lett 17:473–479
Maezono T, Tokumura M, Sekine M, Kawase Y (2011) Hydroxyl radical concentration profile in photo-Fenton oxidation process: Generation and consumption of hydroxyl radicals during the discoloration of azo-dye Orange II. Chemosphere 82:1422–1430
Monteagudo JM, Durán A, López-Almodóvar C (2008) Homogeneus ferrioxalate-assisted solar photo-Fenton degradation of Orange II aqueous solutions. Appl Catal B Environ 82:46–55
Alalm MG, Tawfik A, Ookawara S (2015) Degradation of four pharmaceuticals by solar photo-Fenton process: kinetics and costs estimation. J Environ Chem Eng 3:46–51
Huston PL, Pignatello JJ (1999) Degradation of selected pesticide active ingredients and commercial formulations in water by the photo-assisted Fenton reaction. Water Res 33:1238–1246
Pignatello JJ, Sun Y (1995) Complete oxidation of metolachlor and methyl parathion in water by the photoassisted Fenton reaction. Water Res 29:1837–1844
Evgenidou E, Konstantinou I, Fytianos K, Poulios I (2007) Oxidation of two organophosphorous insecticides by the photo-assisted Fenton reaction. Water Res 41:2015–2027
Pignatello JJ, Chapa G (1994) Degradation of PCBs by ferric ion, hydrogen peroxide and UV light. Environ Toxicol Chem 13(3):423–427
Ruppert G, Bauer R, Heisler G (1993) The photo-Fenton reaction—an effective photochemical wastewater treatment process. J Photochem Photobiol A Chem 73:75–78
Jiang B, Zheng J, Qiu S, Wu M, Zhang Q, Yan Z, Xue Q (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368
Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JG, Graham WG, Graves DB, Hofman-Caris RC, Maric D, Reid JP, Ceriani E, Rivas DF (2016) Plasma-liquid interactions: a review and roadmap. Plasma Sources Sci Technol 25:053002–17
Du CM, Yan JH, Cheron BG (2007) Degradation of 4-Chlorophenol using a gas-liquid gliding arc discharge plasma reactor. Plasma Chem Plasma Process 27:635–646
Burlica R, Kirkpatrick MJ, Locke BR (2006) Formation of reactive species in gliding arc discharges with liquid water. J Electrostat 64:35–43
Burlica R, Shih KY, Locke BR (2010) Formation of H2 and H2O2 in a water-spray gliding arc nonthermal plasma reactor. Ind Eng Chem Res 49:6342–6349
Benstaali B, Moussa D, Addou A, Brisset JL (1998) Plasma treatment of aqueous solutes: some chemical properties of a gliding arc in humid air. Eur Phys J Appl Phys 4:171–179
Moussa D, Abdelmalek F, Benstaali B, Addou A, Hnatiuc E, Brisset JL (2005) Acidity control of the gliding arc treatments of aqueous solutions: application to pollutant abatement and biodecontamination. Eur Phys J Appl Phys 29:189–199
Zhang Y, Zhou M, Lei L (2007) Degradation of 4-chlorophenol in different gas-liquid electrical discharge reactors. Chem Eng J 132:325–333
Robinson JW, Ham M, Balaster AN (1973) Ultraviolet radiation from electrical discharges in water. J Appl Phys 44:72–75
Willberg DM, Lang PS, Höchemer RH, Kratel A, Hoffmann MR (1996) Degradation of 4-chlorophenol, 3, 4-dichloroaniline, and 2, 4, 6-trinitrotoluene in an electrohydraulic discharge reactor. Environ Sci Technol 30:2526–2534
Anpilov AM, Barkhudarov EM, Bark YB, Zadiraka YV, Christofi M, Kozlov YN, Kossyi I, Kop’ev V, Silakov VP, Taktakishvili MI, Temchin SM (2001) Electric discharge in water as a source of UV radiation, ozone and hydrogen peroxide. J Phys D Appl Phys 34:993
Simon A, Anghel SD, Papiu M, Dinu O (2010) Physical and analytical characteristics of an atmospheric pressure argon-helium radiofrequency capacitively coupled plasma. Spectrochim Acta Part B At Spectrosc 65:272–278
Jo YK, Cho J, Tsai TC, Staack D, Kang MH, Roh JH, Shin DB, Cromwell W, Gross D (2014) A non-thermal plasma seed treatment method for management of a seedborne fungal pathogen on rice seed. Crop Sci 54:796–803
Subrahmanyam C, Magureanu M, Laub D, Renken A, Kiwi-Minsker L (2007) Nonthermal plasma abatement of trichloroethylene enhanced by photocatalysis. J Phys Chem C 111:4315–4318
Gharagozalian M, Dorranian D, Ghoranneviss M (2017) Water treatment by the AC gliding arc air plasma. J Theor Appl Phys 11:171–180
Acayanka E, Kuete DS, Kamgang GY, Nzali S, Laminsi S, Ndifon PT (2016) Synthesis, characterization and photocatalytic application of TiO2/SnO2 nanocomposite obtained under non-thermal plasma condition at atmospheric pressure. Plasma Chem Plasma Process 36:799–811
Tarkwa JB, Acayanka E, Jiang B, Oturan N, Kamgang GY, Laminsi S, Oturan MA (2019) Highly efficient degradation of azo dye Orange G using laterite soil as catalyst under irradiation of non-thermal plasma. Appl Catal B Environ 246:211–220
Marković MD, Dojčinović BP, Obradović BM, Nešic J, Natić MM, Tosti TB, Kuraic MM, Manojlović DD (2015) Degradation and detoxification of the 4-chlorophenol by non-thermal plasma-influence of homogeneous catalysts. Sep Purif Technol 154:246–254
Czernichowski A (2001) Glidarc assisted preparation of the synthesis gas from natural and waste hydrocarbons gases. Oil Gas Sci Technol 56:181–198
Du CM, Yan JH, Cheron B (2007) Decomposition of toluene in a gliding arc discharge plasma reactor. Plasma Sources Sci Technol 16:791–797
Lukes P, Clupek M, Babicky V, Sunka P (2008) Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci Technol 17:024012
Calvert JG, Pitts JN (1967) Experimental methods in photochemistry Photochemistry. Wiley, New York, pp 686–814
Furman NH (1962) Iron standard methods of chemical analysis, vol 1. Van Nostrand, Princeton, pp 529–555
Eisenberg GM (1943) Colorimetric determination of hydrogen peroxide Ind. Eng Chem Anal Edn 15:327–328
Acayanka E, Tarkwa JB, Laminsi S (2019) Evaluation of energy balance in a batch and circulating non-thermal plasma reactors during organic pollutant oxidation in aqueous solution. Plasma Chem Plasma Process 39:75–87
Thompson BA, Harteck P, Reeves RRJR (1963) Ultraviolet Absorption Coefficients of CO2, CO, O2, H2O, N2O, NH3, NO, SO2 and CH4 between 1850 and 4000 A. J Geophys Res 68:6431–6433
Bouafia-Chergui S, Oturan N, Khalaf H, Oturan MA (2010) Parametric study on the effect of the ratios [H2O2]/[Fe3+] and [H2O2]/[substrate] on the photo-Fenton degradation of cationic azo dye Basic Blue 41. J Environ Sci Heal Part A 45(5):622–629
Khandelwal DH, Ameta R (2013) Use of photo-fenton reagent for the degradation of Basic Orange 2 in aqueous medium. J Chem Pharm Res 2:39–43
Du Y, Fu QS, Li Y, Su Y (2011) Photodecomposition of 4-chlorophenol by reactive oxygen species in UV/air system. J Hazard Mater 186:491–496
Liu J, Wu JY, Kang CL, Peng F, Liu HF, Yang T, Shi L, Wang HL (2013) Photo-Fenton effect of 4-chlorophenol in ice. J Hazard Mater 261:500–511
Bian W, Song X, Liu D, Zhang J, Chen X (2011) The intermediate products in the degradation of 4-chlorophenol by pulsed high voltage discharge in water. J Hazard Mater 192:1330–1339
Wang Z, Chen X, Ji H, Ma W, Chen C, Zhao J (2010) Photochemical cycling of iron mediated by dicarboxylates: special effect of malonate. Environ Sci Technol 44:263–268
Liotta LF, Gruttadauria M, Di Carlo G, Perrini G, Librando V (2009) Heterogeneous catalytic degradation of phenolic substrates: catalysts activity. J Hazard Mater 162:588–606
Borer P, Hug SJ (2014) Photo-redox reactions of dicarboxylates and α-hydroxydicarboxylates at the surface of Fe(III)(hydr)oxides followed with in situ ATR-FTIR spectroscopy. J Colloid Interface Sci 416:44–53
Baba Y, Yatagai T, Harada T, Kawase Y (2015) Hydroxyl radical generation in the photo-Fenton process: effects of carboxylic acids on iron redox cycling. Chem Eng J 277:229–241
Acknowledgements
The authors are grateful to the International Foundation for Sciences (IFS) (Grant Number: W/4219-1) for the Jenway spectrophotometer granted to SN
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tarkwa, JB., Acayanka, E., Sop, B.T. et al. Effect of Gliding Arc Plasma-Induced UV Light During the Photo-Fenton Oxidation of 4-Chlorophenol in Aqueous Solution. Plasma Chem Plasma Process 41, 989–1007 (2021). https://doi.org/10.1007/s11090-021-10171-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10171-w