Abstract
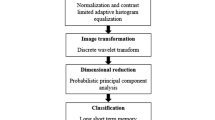
Alzheimer’s disease (AD) is a kind of neurological brain disease. It is an irretrievable, neurodegenerative brain disorder. There are no pills or drugs to cure AD. Therefore, an early diagnosis may help the physician to make accurate analysis and to provide better treatment. With the advent of computational intelligence techniques, machine learning models have made tremendous progress in brain images analysis using MRI, SPECT and PEI. However, accurate analysis of brain scans is an extremely challenging task. The main focus of this paper is to design a Computer Aided Diagnosis (CAD) system using Long-Term Short Memory (LSTM) to improve classification rate and determine suitable attributes that can differentiate AD from Healthy Control (HC) subjects. First, 3D PET images are preprocessed, converted into many groups of 2D images and then grouped into many subsets at certain interval. Subsequently, different features including first order statistical, Gray Level Co-occurrence Matrix and wavelet energy of all sub-bands are extracted from each group, combined and taken as feature vectors. LSTM is designed and employed for classifying PET brain images into HC and AD subjects based on the feature vectors. Finally, the developed system is validated on 18FDG-PET images collected from 188 subjects including 105 HC and 83 AD subjects from ADNI database. Efficacy of the developed CAD system is analyzed using different features. Numerical results revealed that the developed CAD system yields classification accuracy of 98.9% when using combined features, showing outstanding performance.









Similar content being viewed by others
References
Alzheimer’s Disease International (2019) World Alzheimer report 2019: attitudes of dementia, Alzheimer’s disease International, London. https://www.alz.co.uk/research/world-report-2019. Accessed Sept 2019
Patterson C (2018) World Alzheimer report 2018: the state of the art of dementia research: new frontiers. Alzheimer’s Disease International (ADI), London, pp 32–36. https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf. Accessed Sept 2018
Zhan L, Zhou J, Wang Y, Jin Y, Jahanshad N, Prasad G et al (2015) Comparison of nine tractography algorithms for detecting abnormal structural brain networks in Alzheimer’s disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2015.00048
Illán IA, Górriz JM, Ramírez J, Salas-Gonzalez D, López MM, Segovia F et al (2011) 18F-FDG PET imaging analysis for computer aided Alzheimer’s diagnosis. Inf Sci 181(4):903–916. https://doi.org/10.1016/j.ins.2010.10.027
Liu M, Cheng D, Yan W, Alzheimer’s Disease Neuroimaging Initiative (2018) Classification of Alzheimer’s disease by combination of convolutional and recurrent neural networks using FDG-PET images. Front Neuroinform. https://doi.org/10.3389/fninf.2018.00035
López-Gómez C, Ortiz-Ramón R, Mollá-Olmos E, Moratal D, Alzheimer’s Disease Neuroimaging Initiative (2018) ALTEA: a software tool for the evaluation of new biomarkers for alzheimer’s disease by means of textures analysis on magnetic resonance images. Diagnostics 8(3):1–14. https://doi.org/10.3390/diagnostics8030047
Gray KR, Wolz R, Heckemann RA, Aljabar P, Hammers A, Rueckert D, Initiative Alzheimer’s Disease Neuroimaging (2012) Multi-region analysis of longitudinal FDG-PET for the classification of Alzheimer’s disease. NeuroImage 60(1):221–229. https://doi.org/10.1016/j.neuroimage.2011.12.071
Feng C, Elazab A, Yang P, Wang T, Lei B, Xiao X (2018) 3D convolutional neural network and stacked bidirectional recurrent neural network for Alzheimer’s disease diagnosis. In: International workshop on predictive intelligence in medicine, pp 138–146. https://doi.org/10.1007/978-3-030-00320-3_17
Feng C, Elazab A, Yang P, Wang T, Zhou F, Hu H et al (2019) Deep learning framework for Alzheimer’s disease diagnosis via 3D-CNN and FSBi-LSTM. IEEE Access. https://doi.org/10.1109/access.2019.2913847
Liu S, Cai W, Che H, Pujol S, Kikinis R et al (2014) Multimodal neuroimaging feature learning for multiclass diagnosis of Alzheimer’s disease. IEEE Trans Biomed Eng 62(4):1132–1140. https://doi.org/10.1109/TBME.2014.2372011
Suk HI, Lee SW, Shen D, Alzheimer’s Disease Neuroimaging Initiative (2015) Latent feature representation with stacked auto-encoder for AD/MCI diagnosis. Brain Struct Funct 220(2):841–859. https://doi.org/10.1007/s00429-013-0687-3
Li R, Zhang W, Suk HI, Wang L, Li J, Shen D, Ji S (2014) Deep learning based imaging data completion for improved brain disease diagnosis. In: International conference on medical image computing and computer-assisted intervention, pp 305–312. https://koreauniv.pure.elsevier.com/en/publications/deep-learning-based-imaging-data-completion-for-improved-brain-di-2
Kwon GR, Gupta Y, Lama RK (2019) Prediction and classification of Alzheimer’s disease based on combined features from apolipoprotein-E genotype, cerebrospinal fluid, MR, and FDG-PET imaging biomarkers. Front Comput Neurosci 13(72):1–18. https://doi.org/10.3389/fncom.2019.00072
Garali I, Adel M, Bourennane S, Guedj E (2018) Histogram-based features selection and volume of interest ranking for brain PET image classification. IEEE J Transl Eng Health Med. https://doi.org/10.1109/jtehm.2018.2796600
Tong T, Gray K, Gao Q, Chen L, Rueckert D, Alzheimer’s Disease Neuroimaging Initiative (2017) Multi-modal classification of Alzheimer’s disease using nonlinear graph fusion. Pattern Recognit. https://doi.org/10.1016/j.patcog.2016.10.009
Alzheimer’s Disease Neuroimaging initiative PET technical procedures manual. https://adni.loni.usc.edu/wpcontent/uploads/2010/09/PET_PIB_Tech_Procedures_Manual_Suppl_v1.3.pdf. Accessed 10 May 2007
ADNI 2 PET technical procedures manual for FDG and AV-45 ADNI 2 PET technical procedures manual AV-45 (Florbetapir F 18) & FDG. https://adni.loni.usc.edu/wp-content/uploads/2010/05/ADNI2_PET_Tech_Manual_0142011.pdf. Accessed 14 Jan 2011
ADNI-GO PET technical procedures manual for FDG and AV-45 ADNI-GO.PET technical procedure manual. https://adni.loni.usc.edu/wp-content/uploads/2010/05/ADNIGO_PET_Tech_Manual_01142011.pdf. Accessed 14 Jan 2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherin, A., Rajeswari, R. Computer-Aided Diagnosis System for Alzheimer’s Disease Using Positron Emission Tomography Images. Interdiscip Sci Comput Life Sci 13, 433–442 (2021). https://doi.org/10.1007/s12539-020-00409-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-020-00409-0