Abstract
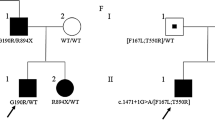
We report a multiplex family with extended multisystem neurological phenotype associated with a CRYAB variant. Two affected siblings were evaluated with whole exome sequencing, muscle biopsy, laser microdissection, and mass spectrometry-based proteomic analysis. Both patients and their mother manifested a combination of early-onset cataracts, cardiomyopathy, cerebellar ataxia, optic atrophy, cognitive impairment, and myopathy. Whole exome sequencing identified a heterozygous c.458C>T variant mapped to the C-terminal extension domain of the Alpha-crystallin B chain, disrupting its function as a molecular chaperone and its ability to suppress protein aggregation. In accordance with the molecular findings, muscle biopsies revealed subsarcolemmal deposits that appeared dark with H&E and trichrome staining were negative for the other routine histochemical staining and for amyloid with the Congo-red stain. Electron microscopy demonstrated that the deposits were composed of numerous parallel fibrils. Laser microdissection and mass spectrometry-based proteomic analysis revealed that the inclusions are almost exclusively composed of crystallized chaperones/heat shock proteins. Moreover, a structural model suggests that Ser153 could be involved in monomer stabilization, dimer association, and possible binding of partner proteins. We propose that our report potentially expands the complex phenotypic spectrum of alpha B-crystallinopathies with possible effect of a CRYAB variant on the central nervous system.





Similar content being viewed by others
References
Augusteyn RC (2004) α-crystallin: a review of its structure and function. Clin Exp Optom 87:356–366
van der Smagt JJ, Vink A, Kirkels JH, Nelen M, ter Heide H, Molenschot MM et al (2014) Congenital posterior pole cataract and adult onset dilating cardiomyopathy: expanding the phenotype of αB-crystallinopathies. Clin Genet 85:381–385
Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE (2011) N-terminal domain of alpha B-crytallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci U S A 108(16):6409–6414
Braun N, Zacharias M, Peschek J, Kastenmüller A, Zou J, Hanzlik M et al (2011) Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc Natl Acad Sci U S A 108:20491–20496
Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, Chateau D, Chapon F, Tomé F, Dupret JM, Paulin D, Fardeau M (1998) A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20:92–95
Selcen D, Engel AG (2003) Myofibrillar myopathy caused by novel dominant negative αB-crystallin mutations. Ann Neurol 54:804–810
Reilich P, Schoser B, Schramm N, Krause S, Schessl J, Kress W, Müller-Höcker J, Walter MC, Lochmuller H (2010) The p.G154S mutation of the alpha-B crystallin gene (CRYAB) causes late-onset distal myopathy. Neuromuscul Disord 20:255–259
Del Bigio MR, Chudley AE, Sarnat HB, Campbell C, Goobie S, Chodirker BN et al (2011) Infantile muscular dystrophy in Canadian Aboriginals is an αB-crystallinopathy. Ann Neurol 69:866–871
Sacconi S, Féasson L, Antoine JC, Pécheux C, Bernard R, Cobo AM, Casarin A, Salviati L, Desnuelle C, Urtizberea A (2012) A novel CRYAB mutation resulting in multisystemic disease. Neuromuscul Disord 22:66–72
Marcos AT, Amorós D, Muñoz-Cabello B, Galán F, Infante ER, Alcaraz-Mas L, Navarro-Pando JM (2020) A novel dominant mutation in CRYAB gene leading to a severe phenotype with childhood onset. Mol Genet Genomic Med 8(8):e1290
Harel T, Hacohen N, Shaag A, Gomori M, Singer A, Elpeleg O, Meiner V (2017) Homozygous null variant in CRADD, encoding an adaptor protein that mediates apoptosis, is associated with lissencephaly. Am J Med Genet A 173:2539–2544
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinforma Oxf Engl 25:1754–1760
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup (2009) 1000 genome project data processing subgroup. Bioinformatics 25:2078–2079
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T et al (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536:285–291
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi BB, Wang Q et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581(7809):434–443
Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ et al (2013) Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 34:57–65
Choi Y, Chan AP (2015) PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31:2745–2747
Kortemme T, Kim DE, Baker D (2004) Computational alanine scanning of protein-protein interfaces. Sci STKE 219:12
Raju I, Abraham EC (2013) Mutants of human αB-crystallin cause enhanced protein aggregation and apoptosis in mammalian cells: influence of co-expression of HspB1. Biochem Biophys Res Commun 430:107–112
Pilotto A, Marziliano N, Pasotti M, Grasso M, Costante AM (2006) Arbustini E alphaB-crystallin mutation in dilated cardiomyopathies: low prevalence in a consecutive series of 200 unrelated probands. Biochem Biophys Res Commun 346:1115–1117
Semmler AL, Sacconi S, Bach JE, Liebe C, Bürmann J, Kley RA, Ferbert A, Anderheiden R, van den Bergh P, Martin JJ, de Jonghe P, Neuen-Jacob E, Müller O, Deschauer M, Bergmann M, Schröder JM, Vorgerd M, Schulz JB, Weis J, Kress W, Claeys KG (2014) Unusual multisystemic involvement and novel BAG3 mutation revealed by NGS screening in a large cohort of myofibrillar myopathies. Orphanet J Rare Dis 9:121
Kirbach BB, Golenhofen N (2011) Differential expression and induction of small heat shock proteins in rat brain and cultured hippocampal neurons. J Neurosci Res 89:162–175
Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW (1994) Expression of aB-crystallin in Alzheimer’s disease. Acta Neuropathol 87:155–160
Renkawek K, Stege GJ, Bosman GJ (1999) Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport 10:2273–2276
Iwaki A, Iwaki T, Goldman JE, Ogomori K, Tateishi J, Sakaki Y (1992) Accumulation of alpha B-crystallin in brains of patients with Alexander’s disease is not due to an abnormality of the 5’-flanking and coding sequence of the genomic DNA. Neurosci Lett 140:89–92
Iwaki T, Wisniewski T, Iwaki A, Iwaki A, Corbin E, Tomokane N et al (1992) Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. Am J Pathol 140:345–356
Selcen D (2011) Myofibrillar myopathies. Neuromuscul Disord 21:161–171
Tajsharghi H, Thornell LE, Lindberg C, Lindvall B, Henriksson CG, Oldfors A (2003) Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann Neurol 54:494–500
Bohlega S, Lach B, Meyer BF, Al Said Y, Kambouris M, Al Homsi M et al (2003) Autosomal dominant hyaline body myopathy: clinical variability and pathologic findings. Neurology 61:1519–1523
Barohn RJ, Brumback RA, Mendell JR (1994) Hyaline body myopathy. Neuromuscul Disord 4:257–262
Arrigo AP (2013) Human small heat shock proteins: protein interactomes of homo-and hetero-oligomeric complexes: an update. FEBS Lett 587:1959–1969
Dimauro I, Antonioni A, Mercatelli N, Caporossi D (2018) The role of αB-crystallin in skeletal and cardiac muscle tissues. Cell Stress Chaperones 23:491–505
Clark JI (1860) Functional sequences in human alphaB crystallin. Biochim Biophys Acta 2016:240–245
Inagakia N, Hayashia T, Arimura T, Koga Y, Takahashi M, Shibata H et al (2006) Alpha B-crystallin mutation in dilated cardiomyopathy. Biophys Res Commun 342:379–386
Funding
Work in CGB’s lab is provided by intramural funds of NINDS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies were in accordance with the ethical standards of the institutional committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadeh, M., Rahat, D., Meiner, V. et al. Multi-system neurological disorder associated with a CRYAB variant. Neurogenetics 22, 117–125 (2021). https://doi.org/10.1007/s10048-021-00640-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-021-00640-x