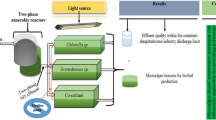
Abstract
The aim of this study is to cultivate Spirulina platensis and Scenedesmus obliquus microalgae in consortia using effluents of cattle waste anaerobic treatment in order to give possibilities to the production of microalgae biomass to biorefineries uses. The biomasses obtained were characterized to evaluate the potential for the production of biofuels and other bioproducts. The effluent was used in sterile and non-sterile conditions to better understand the influence of other microorganisms in N and P removal. The biomass obtained with the addition of 10% of sterile effluent in Zarrouk media (20%) presented 44.12 and 34.62% of carbohydrates, using Spirulina platensis in monoculture or the 50%/50% consortia of Spirulina and Scenedesmus, respectively, this biomass presenting the potential to be used to bioethanol production. Nitrogen and phosphorous removal were higher in non-sterile conditions and reached 92.7 and 49.66% of nitrogen and phosphorous removal, respectively, using the consortia and with the addition of 30% effluent in the media. The cultivation of microalgae in a consortium may be used to assist the treatment of water concurrently with the production of biomass to different applications.





Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
References
Vadiveloo A, Nwoba EG, Moheimani NR (2019) Viability of combining microalgae and macroalgae cultures for treating anaerobically digested piggery effluent. J Environ Sci 82:132–144. https://doi.org/10.1016/j.jes.2019.03.003
Souza MP De, Hoeltz M, Gressler PD et al (2019) Potential of microalgal bioproducts: general perspectives and main challenges. Waste Biomass Valori 10:2139–21560. https://doi.org/10.1007/s12649-018-0253-6
Vieira Salla AC, Margarites AC, Seibel FI, Holz LC, Brião VB, Bertolin TE, Colla LM, Costa JAV (2016) Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour Technol 209:133–141. https://doi.org/10.1016/j.biortech.2016.02.069
Miranda JR, Passarinho PC, Gouveia L (2012) Bioethanol production from Scenedesmus obliquus sugars: the influence of photobioreactors and culture conditions on biomass production. Appl Microbiol Biotechnol 96:555–564. https://doi.org/10.1007/s00253-012-4338-z
Sarkar D, Shimizu K (2015) An overview on biofuel and biochemical production by photosynthetic microorganisms with understanding of the metabolism and by metabolic engineering together with efficient cultivation and downstream processing. Bioresour Bioprocess 2:17. https://doi.org/10.1186/s40643-015-0045-9
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Popp J, Lakner Z, Harangi-rákos M, Fári M (2014) The effect of bioenergy expansion: food, energy, and environment. Renew Sust Energ Rev 32:559–578. https://doi.org/10.1016/j.rser.2014.01.056
Colla LM, Margarites F et al (2019) Waste biomass and blended bioresources in biogas production. Improv Biogas Prod 01:1–23. https://doi.org/10.1007/978-3-030-10516-7_1
Myeong H, Hoon C, Bae H (2017) Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production. Bioresour Technol 233:44–50. https://doi.org/10.1016/j.biortech.2017.02.040
Man H, Liu H, Xiao Q, Deng F, Yu Q, Wang K, Yang Z, Wu Y, He K, Hao J (2018) How ethanol and gasoline formula changes evaporative emissions of the vehicles. Appl Energy 222:584–594. https://doi.org/10.1016/j.apenergy.2018.03.109
Daylan B, Ciliz N (2016) Life cycle assessment and environmental life cycle costing analysis of lignocellulosic bioethanol as an alternative transportation fuel. Renew Energy 89:578–587. https://doi.org/10.1016/j.renene.2015.11.059
Mohammadi M, Mowla D, Esmaeilzadeh F, Ghasemi Y (2018) Cultivation of microalgae in a power plant wastewater for sulfate removal and biomass production: a batch study. J Environ Chem Eng 6:2812–2820. https://doi.org/10.1016/J.JECE.2018.04.037
Jebali A, Acién FG, Sayadi S, Molina-Grima E (2018) Utilization of centrate from urban wastewater plants for the production of Scenedesmus sp. in a raceway-simulating reactor. J Environ Manag 211:112–124. https://doi.org/10.1016/j.jenvman.2018.01.043
Rempel A, Biolchi G, Antunes AC et al (2020) Cultivation of microalgae in media added of emergent pollutants and effect on growth, chemical composition, and use of biomass to enzymatic hydrolysis. Bioenergy Res 14:265–277. https://doi.org/10.1007/s12155-020-10177-w
Rempel A, Gutkoski JP, Nazari MT, Biolchi GN, Cavanhi VAF, Treichel H, Colla LM (2021) Current advances in microalgae-based bioremediation and other technologies for emerging contaminants treatment. Sci Total Environ 772:144918. https://doi.org/10.1016/j.scitotenv.2020.144918
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193. https://doi.org/10.1016/j.biortech.2010.06.139
Zaparoli M, Ziemniczak FG, Mantovani L et al (2020) Cellular stress conditions as a strategy to increase carbohydrate productivity in Spirulina platensis. Bioenergy Res 13:1221–1234. https://doi.org/10.1007/s12155-020-10133-8
Wang S, Stiles AR, Guo C, Liu C (2015) Harvesting microalgae by magnetic separation: a review. Algal Res 9:178–185. https://doi.org/10.1016/j.algal.2015.03.005
Harun R, Danquah K, Forde GM (2010) Microalgal biomass as a fermentation feedstock for bioethanol production. J Chem Technol Biotechnol 85:199–203. https://doi.org/10.1002/jctb.2287
Magro FG, Margarites AC, Reinehr CO, Gonçalves GC, Rodigheri G, Costa JAV, Colla LM (2017) Spirulina platensis biomass composition is influenced by the light availability and harvest phase in raceway ponds. Environ Technol 3330:1–10. https://doi.org/10.1080/09593330.2017.1340352
Ruiz-Martinez A, Serralta J, Pachés M, Seco A, Ferrer J (2014) Mixed microalgae culture for ammonium removal in the absence of phosphorus: effect of phosphorus supplementation and process modeling. Process Biochem 49:2249–2257. https://doi.org/10.1016/j.procbio.2014.09.002
Davis RW, Siccardi AJ, Huysman ND et al (2015) Growth of mono- and mixed cultures of Nannochloropsis salina and Phaeodactylum tricornutum on struvite as a nutrient source. Bioresour Technol 198:577–585. https://doi.org/10.1016/j.biortech.2015.09.070
Almomani FA, Örmeci B (2016) Performance of Chlorella Vulgaris, Neochloris Oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent and centrate. Ecol Eng 95:280–289. https://doi.org/10.1016/j.ecoleng.2016.06.038
Marazzi F, Bellucci M, Fantasia T, Ficara E, Mezzanotte V (2020) Interactions between microalgae and bacteria in the treatment of wastewater from milk whey processing. Water 12:297. https://doi.org/10.3390/w12010297
Wang Y, Wang S, Sun L, Sun Z, Li D (2020) Screening of a Chlorella-bacteria consortium and research on piggery wastewater purification. Algal Res 47:101840. https://doi.org/10.1016/j.algal.2020.101840
Pires JCM, Martins FG, Simões M (2012) Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew Sust Energ Rev 16:3043–3053. https://doi.org/10.1016/j.rser.2012.02.055
Batista AP, Ambrosano L, Graça S, Sousa C, Marques PASS, Ribeiro B, Botrel EP, Castro Neto P, Gouveia L (2015) Combining urban wastewater treatment with biohydrogen production—an integrated microalgae-based approach. Bioresour Technol 184:230–235. https://doi.org/10.1016/j.biortech.2014.10.064
Posadas E, Morales M, Gomez C et al (2015) Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem Eng J 265:239–248. https://doi.org/10.1016/j.cej.2014.12.059
Koreiviene J, Valčiukas R, Karosiene J, Baltrenas P (2014) Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, CO2 mitigation and algae biomass feasibility for lipid production. J Environ Eng Landsc Manag 22:105–114. https://doi.org/10.3846/16486897.2013.911182
Castro YA, Ellis JT, Miller CD, Sims RC (2015) Optimization of wastewater microalgae saccharification using dilute acid hydrolysis for acetone, butanol, and ethanol fermentation. Appl Energy 140:14–19. https://doi.org/10.1016/j.apenergy.2014.11.045
Choong YJ, Yokoyama H, Matsumura Y, Lam MK, Uemura Y, Dasan YK, Kadir WNA, Lim JW (2020) The potential of using microalgae for simultaneous oil removal in wastewater and lipid production. Int J Environ Sci Technol 17:2755–2766. https://doi.org/10.1007/s13762-020-02701-4
Zarrouk C (1966) Contribution to the study of a Cyanophycea: influence of various physical and chemical factors on the growth and photosynthesis of Spirulina platensis (Thesis). University of Paris
De Morais MG, Costa JAV (2007) Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol Lett 29:1349–1352. https://doi.org/10.1007/s10529-007-9394-6
Lv J, Guo J, Feng J, Liu Q, Xie S (2016) A comparative study on flocculating ability and growth potential of two microalgae in simulated secondary effluent. Bioresour Technol 205:111–117. https://doi.org/10.1016/j.biortech.2016.01.047
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association/American Water Works Association/ Water Environment Federation, Washington DC
AOAC (2000) Official methods of analysis of AOAC International, 17th edn. Association of official analytical chemists, Gaithersburg
Gómez-Serrano C, Morales-Amaral MM, Acién FG, Escudero R, Fernández-Sevilla JM, Molina-Grima E (2015) Utilization of secondary-treated wastewater for the production of freshwater microalgae. Appl Microbiol Biotechnol 99:6931–6944. https://doi.org/10.1007/s00253-015-6694-y
Costa JAV, Colla LM, Filho PD (2002) Modelling of Spirulina platensis growth in fresh water using response surface methodology. World J Microbiol Biotechnol 18:603–607. https://doi.org/10.1023/A:1016822717583
Dubois M, Gilles K, Hamilton J et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Lowry OH (1951) Protein measurement with the Folin-Phenol reagent. J Biol Chem 193:265–275
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2a edn. McGraw-Hill, Singapore isbn:0-07-Y66601-6
Margarites AC, Volpato N, Araújo E, Cardoso LG, Bertolin TE, Colla LM, Costa JAV (2016) Spirulina platensis is more efficient than Chlorella homosphaera in carbohydrate productivity. Environ Technol 3330:1–8. https://doi.org/10.1080/09593330.2016.1254685
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19:235–240. https://doi.org/10.1016/j.copbio.2008.05.007
Tuantet K, Janssen M, Temmink H, Zeeman G, Wijffels RH, Buisman CJN (2014) Microalgae growth on concentrated human urine. J Appl Phycol 26:287–297. https://doi.org/10.1007/s10811-013-0108-2
He PJ, Mao B, Shen CM, Shao LM, Lee DJ, Chang JS (2013) Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol 129:177–181. https://doi.org/10.1016/j.biortech.2012.10.162
Sniffen KD, Sales CM, Olson MS (2018) The fate of nitrogen through algal treatment of landfill leachate. Algal Res 30:50–58. https://doi.org/10.1016/j.algal.2017.12.010
Hultberg M, Lind O, Birgersson G, Asp H (2017) Use of the effluent from biogas production for cultivation of Spirulina. Bioprocess Biosyst Eng 40:625–631. https://doi.org/10.1007/s00449-016-1726-2
Morales-Amaral M d M, Gómez-Serrano C, Acién FG et al (2015) Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res 12:99–108. https://doi.org/10.1016/j.algal.2015.08.020
Gamfeldt L, Hillebrand H (2011) Effects of total resources, resource ratios, and species richness on algal productivity and evenness at both metacommunity and local scales. PLoS One 6:e21972. https://doi.org/10.1371/journal.pone.0021972
Behl S, Donval A, Stibor H (2011) The relative importance of species diversity and functional group diversity on carbon uptake in phytoplankton communities. Limnol Oceanogr 56:683–694. https://doi.org/10.4319/lo.2011.56.2.0683
Gonçalves AL, Pires JCM, Simões M (2016) A review on the use of microalgal consortia for wastewater treatment. Algal Res 24:403–415. https://doi.org/10.1016/j.algal.2016.11.008
Hena S, Znad H, Heong KT, Judd S (2018) Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res 128:267–277. https://doi.org/10.1016/j.watres.2017.10.057
de Mendonça HV, Ometto JPHB, Otenio MH, Marques IPR, dos Reis AJD (2018) Microalgae-mediated bioremediation and valorization of cattle wastewater previously digested in a hybrid anaerobic reactor using a photobioreactor: comparison between batch and continuous operation. Sci Total Environ 633:1–11. https://doi.org/10.1016/j.scitotenv.2018.03.157
Paddock MB, Fernández-Bayo JD, VanderGheynst JS (2020) The effect of the microalgae-bacteria microbiome on wastewater treatment and biomass production. Appl Microbiol Biotechnol 104:893–905. https://doi.org/10.1007/s00253-019-10246-x
Rada-Ariza AM, Lopez-Vazquez CM, van der Steen NP, Lens PNL (2017) Nitrification by microalgal-bacterial consortia for ammonium removal in flat panel sequencing batch photo-bioreactors. Bioresour Technol 245:81–89. https://doi.org/10.1016/j.biortech.2017.08.019
Scherer MD, de Oliveira AC, Filho FJCM, Ugaya CML, Mariano AB, Vargas JVC (2017) Environmental study of producing microalgal biomass and bioremediation of cattle manure effluents by microalgae cultivation. Clean Techn Environ Policy 19:1745–1759. https://doi.org/10.1007/s10098-017-1361-x
Luo L, Ren H, Pei X, Xie G, Xing D, Dai Y, Ren N, Liu B (2019) Simultaneous nutrition removal and high-efficiency biomass and lipid accumulation by microalgae using anaerobic digested effluent from cattle manure combined with municipal wastewater. Biotechnol Biofuels 12:1–15. https://doi.org/10.1186/s13068-019-1553-1
Makut BB, Das D, Goswami G (2019) Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: a sustainable approach. Algal Res 37:228–239. https://doi.org/10.1016/j.algal.2018.11.020
Choi KJ, Han TH, Yoo G, Cho MH, Hwang SJ (2018) Co-culture consortium of Scenedesmus dimorphus and nitrifiers enhances the removal of nitrogen and phosphorus from artificial wastewater. KSCE J Civ Eng 22:3215–3221. https://doi.org/10.1007/s12205-017-0730-7
Acknowledgements
The authors are thankful to the University of Passo Fundo for the scholarships.
Author information
Authors and Affiliations
Contributions
Francisco G. Magro: conceptualization, methodology, formal analysis and investigation, and writing. João F. Freitag and André Bergoli: methodology and writing. Jorge A.V. Costa: review. Luciane Maria Colla: conceptualization, writing, reviewing and editing, and supervision.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors declare consent for publication of this article, being represented by the author for correspondence.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1110 kb).
Rights and permissions
About this article
Cite this article
Magro, F.G., Freitag, J.F., Bergoli, A. et al. Microalgae Consortia for Post-treating Effluent of Anaerobic Digestion of Cattle Waste and Evaluation of Biochemical Composition of Biomass. Bioenerg. Res. 15, 371–384 (2022). https://doi.org/10.1007/s12155-021-10270-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10270-8