Abstract
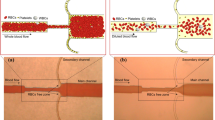
Recently, microfluidic bioreactors that trap injected megakaryocytes (MKs) by application of fluid force to them have been proposed as small test benches to evaluate the in vitro platelet production process. However, making a flow rate constant after trapping MKs remains a challenge and bottleneck because the cross-sectional area of the microchannel decreases due to the trapped MKs. Therefore, we present a microfluidic bioreactor containing a three-dimensional microchannel that has been designed based on the cell size distribution of immortalized megakaryocyte cell lines (imMKCLs). As results, we succeeded in trapping imMKCLs with small variations in the cross-sectional area along the flow path. Through experiments on on-chip production of platelet-like particles (PLPs) for 12 h using imMKCLs derived from human-induced pluripotent stem cells, we found that the average number of the total produced PLPs per imMKCLs was 23, 24, 16, and 14 when the applied pressures was 10, 50, 100 and 200 kPa, respectively. From these results, we confirmed that the proposed microfluidic bioreactor can be applied as a test bench for evaluating of the on-chip PLP production.





Similar content being viewed by others
References
Blin A, Go AL, Magniez A, Poirault CS, Teste B, Sicot G, Nguyen KA, Hamdi FS, Reyssat M, Baruch D (2016) Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics. Sci Rep 6:21700
Campbell JW (1995) The lognormal distribution as a model for bio-optical variability in the sea. J Geophys Res 100(C7):13237–13254
Campbell J, Yentsch C, Cucci T (1989) Variance within homogeneous phytoplankton populations, III: analysis of natural populations. Cytometry 10(5):605–611
Ichikawa A, Sakuma S, Sugita M, Shoda T, Tamakoshi T, Akagi S, Arai F (2014) On-chip enucleation of an oocyte by untethered microrobots. J Micromech Microeng 24(9):095004
Ito Y, Nakamura S, Sugimoto N, Shigemori T, Kato Y, Ohno M, Sakuma S, Ito K, Kumon H, Hirose H, Okamoto H, Nogawa M, Iwasaki M, Kihara S, Fujio K, Matsumoto T, Higashi N, Hashimoto K, Sawaguchi A, Harimoto K, Nakagawa M, Yamamoto T, Handa M, Watanabe N, Nishi E, Arai F, Nishimura S, Eto K (2018) Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell 174:636–648
Maruo S, Nakamura O, Kawata S (1997) Three-dimensi-onal microfabrication with two-photon-absorbed photopolymerization. Opt Lett 22(2):132–134
Moreau T, Evans AL, Vasquez L, Tijssen MR, Yan Y, Trotter MW, Howard D, Colzani M, Arumugam M, Wu WH, Dalby A, Lampela R, Bouet G, Hobbs CM, Pask DC, Payne H, Ponomaryov T, Brill A, Soranzo N, Ouwehand WH, Pedersen RA, Ghevaert C (2016) Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun 7:11208
Nakagawa Y, Nakamura S, Nakajima M, Endo H, Dohda T, Takayama N, Nakauchi H, Arai F, Fukuda T, Eto K (2013) Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp Hematol 41(8):742–748
Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, Fujita K, Koike T, Harimoto K, Dohda T, Watanabe A, Okita K, Takahashi N, Sawaguchi A, Yamanaka S, Nakauchi H, Nishimura S, Eto K (2014) Expandable megakaryocyte cell Lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 14(4):535–548
Nishimura S, Nagasaki M, Kunishima S, Sawaguchi A, Sakata A, Sakaguchi H, Ohmori T, Manabe I, Italiano JE, Ryu T, Takayama N, Komuro I, Kadowaki T, Eto K, Nagai R (2015) IL-1\(\alpha\) induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol 209(3):453–466
Pallotta I, Lovett M, Kaplan DL, Balduini A (2011) Three-Dimensional System for the In Vitro Study of Megakaryocytes and Functional Platelet production using silk-based vascular tubes. Tissue Eng Part C Methods 17(12):1223–1232
Seo H, Chen SJ, Hashimoto K, Endo H, Nishi Y, Ohta A, Yamamoto T, Hotta A, Sawaguchi A, Hayashi H, Koseki N, Murphy GJ, Fukuda K, Sugimoto N, Eto K (2018) A \(\beta\)1-tubulinbased megakaryocyte maturation reporter system identifies novel drugs that promote platelet production. Blood Adv 2(17):2262–2272
Shallan AI, Smejkal P, Corban M, Guijt RM, Breadmore MC (2014) Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Anal Chem 86(6):3124–3130
Sim X, Poncz M, Gadue P, French DL (2016) Understanding platelet generation from megakaryocytes: implications for in vitro-derived platelets. Nat Commun 127:1227–1233
Stroncek DF, Rebulla P (2007) Platelet transfusions. Lancet 370:427–438
Takayama N, Nishimura S, Nakamura S, Shimizu T, Ohnishi R, Endo H, Yamaguchi T, Otsu M, Nishimura K, Nakanishi M, Sawaguchi A, Nagai R, Takahashi K, Yamanaka S, Nakauchi H, Eto K (2010) Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med 207(13):2817–2830
Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, Feng Q, Lu S, Lanza R, Neeves KB, Weitz DA, Italiano JE (2014) Platelet bioreactor-on-a-chip. Blood 124(12):1857–1867
Waits C, Morgan B, Kastantin M, Ghodssi R (2005) Microfabrication of 3D silicon MEMS structures using gray-scale lithography and deep reactive ion etching. Sens Actuat A 119(1):245–253
Walczak R, Adamski K (2015) Inkjet 3D printing of microfluidic structures on the selection of the printer towards printing your own microfluidic chips. J Micromech Microeng 25(8):085013
Williamson LM, Devine DV (2013) Challenges in the management of the blood supply. Lancet 381(9880):25–31
Zhou W, Kuebler SW, Braun KL, Yu T, Cammack JK, Ober CK, Perry JW, Marder SR (2002) An efficient two-photon-generated photoacid applied to positive-tone 3D microfabrication. Science 296(5570):1106–1109
Acknowledgements
This work was supported in part by the Highway Program for Realization of Regenerative Medicine (JP17bm-0504008, K.E.), Practical Applications of Regenerative Medicine (JP17bk0104039, K.E.), Core Center for iPS Cell Research (JP17bm0104001, N.S. and K.E.) from the Japan Agency for Medical Research and Development (AMED), and by a Grant-in-Aid for scientific research (15H03005, K.E.; 19J23654, H.K.) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
SS, HK, and FA conceived the concept of the 3D microchannel reflecting cell size distribution. SS, HK, YK, and FA designed and constructed the fluidic system. SS and HK designed and fabricated the microfluidic chip. SN and KE prepared the cell samples. SS, HK, and SN designed and conducted the experiments. SS, HK, and SN conducted data analyses. KE and FA supervised this work. All authors participated in preparing the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1 (mp4 2029 KB)
Supplementary material 2 (mp4 1538 KB)
Supplementary material 3 (mp4 4231 KB)
Supplementary material 4 (mp4 1864 KB)
Rights and permissions
About this article
Cite this article
Sakuma, S., Kumon, H., Nakamura, S. et al. Three-dimensional microchannel reflecting cell size distribution for on-chip production of platelet-like particles. Microfluid Nanofluid 25, 36 (2021). https://doi.org/10.1007/s10404-021-02433-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-021-02433-y