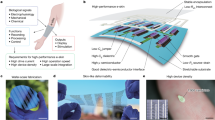
Abstract
Monitoring the flow rate, cumulative loss and temperature of sweat can provide valuable physiological insights for the diagnosis of thermoregulatory disorders and illnesses related to heat stress. However, obtaining accurate, continuous estimates of these parameters with high temporal resolution remains challenging. Here, we report a platform that can wirelessly measure sweat rate, sweat loss and skin temperature in real time. The approach combines a short, straight fluid passage to capture sweat as it emerges from the skin with a flow sensor that is based on a thermal actuator and precision thermistors, and that is physically isolated from, but thermally coupled to, the sweat. The platform transfers data autonomously using a Bluetooth Low Energy system on a chip. Our approach can also be integrated with advanced microfluidic systems and colorimetric chemical reagents for the measurement of pH and the concentration of chloride, creatinine and glucose in sweat.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Supporting data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Custom-developed firmware for BLE SoCs and Android applications (user interfaces) for use on smartphones are available from the corresponding author upon reasonable request. All requests for source code will be reviewed by the corresponding author to verify whether the request is subject to any intellectual property or confidentiality obligations.
References
Baker, L. B. Sweating rate and sweat sodium concentration in athletes: a review of methodology and intra/interindividual variability. Sports Med. 47, 111–128 (2017).
Gambhir, S. S., Ge, T. J., Vermesh, O. & Spitler, R. Toward achieving precision health. Sci. Transl. Med. 10, eaao3612 (2018).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport and biosensing implications. Biomicrofluidics 9, 031301 (2015).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Reeder, J. T. et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis and thermography in aquatic settings. Sci. Adv. 5, eaau6356 (2019).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Baker, L. B., Stofan, J. R., Hamilton, A. A. & Horswill, C. A. Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise. J. Appl. Physiol. 107, 887–895 (2009).
Maughan, R. J. et al. Water balance and salt losses in competitive football. Int. J. Sport Nutr. Exerc. Metab. 17, 583–594 (2007).
Williams, C. A. & Blackwell, J. Hydration status, fluid intake and electrolyte losses in youth soccer players. Int. J. Sports Physiol. Perform. 7, 367–374 (2012).
Al-omari, M. et al. A portable optical human sweat sensor. J. Appl. Phys. 116, 183102 (2014).
Bandodkar, A. J. & Wang, J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371 (2014).
Dam, V. A. T., Zevenbergen, M. A. G. & van Schaijk, R. Toward wearable patch for sweat analysis. Sens. Actuators B Chem. 236, 834–838 (2016).
Bain, A. R., Deren, T. M. & Jay, O. Describing individual variation in local sweating during exercise in a temperate environment. Eur. J. Appl. Physiol. 111, 1599–1607 (2011).
Patterson, M. J., Galloway, S. D. R. & Nimmo, M. A. Variations in regional sweat composition in normal human males. Exp. Physiol. 85, 869–875 (2000).
Matzeu, G., Fay, C., Vaillant, A., Coyle, S. & Diamond, D. A wearable device for monitoring sweat rates via image analysis. IEEE Trans. Biomed. Eng. 63, 1672–1680 (2016).
Choi, J., Ghaffari, R., Baker, L. B. & Rogers, J. A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 4, eaar3921 (2018).
Francis, J., Stamper, I., Heikenfeld, J. & Gomez, E. F. Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement. Lab Chip 19, 178–185 (2019).
Iftekhar, A. T., Ho, J. C.-T., Mellinger, A. & Kaya, T. 3D modeling and characterization of a calorimetric flow rate sensor for sweat rate sensing applications. J. Appl. Phys. 121, 094505 (2017).
Brueck, A., Iftekhar, T., Stannard, B. A., Yelamarthi, K. & Kaya, T. A real-time wireless sweat rate measurement system for physical activity monitoring. Sensors 18, 533 (2018).
Farrell, P. M. et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J. Pediatr. 153, S4–S14 (2008).
Moyer, J., Wilson, D., Finkelshtein, I., Wong, B. & Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14, 398–402 (2012).
Robinson, S. & Robinson, A. H. Chemical composition of sweat. Physiol. Rev. 34, 202–220 (1954).
Bass, D. E. & Dobalian, I. T. Ratio between true and apparent creatinine in sweat. J. Appl. Physiol. 5, 555–558 (1953).
Al-Tamer, Y. Y., Hadi, E. A. & Al-Badrani, I. E. I. Sweat urea, uric acid and creatinine concentrations in uraemic patients. Urol. Res. 25, 337–340 (1997).
Harvey, C. J., LeBouf, R. F. & Stefaniak, A. B. Formulation and stability of a novel artificial human sweat under conditions of storage and use. Toxicol. In Vitro 24, 1790–1796 (2010).
Huang, C.-T., Chen, M.-L., Huang, L.-L. & Mao, I.-F. Uric acid and urea in human sweat. Chin. J. Physiol. 45, 109–115 (2002).
Brinkman, J. E. & Sharma, S. Physiology, Metabolic Alkalosis (StatPearls Publishing, 2019).
Patterson, M. J., Galloway, S. D. R. & Nimmo, M. A. Effect of induced metabolic alkalosis on sweat composition in men. Acta Physiol. Scand. 174, 41–46 (2002).
Choi, J. et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 4, 379–388 (2019).
Zhang, Y. et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab Chip 19, 1545–1555 (2019).
Emrich, H. M. et al. Sweat composition in relation to rate of sweating in patients with cystic fibrosis of the pancreas. Pediatr. Res. 2, 464–478 (1968).
Ohara, K. Chloride concentration in sweat; its individual, regional, seasonal and some other variations, and interrelations between them. Jpn J. Physiol 16, 274–290 (1966).
Coyle, S. et al. Textile sensors to measure sweat pH and sweat-rate during exercise. In Proc. 3rd International ICST Conference on Pervasive Computing Technologies for Healthcare 1–6 https://doi.org/10.4108/ICST.PERVASIVEHEALTH2009.5957 (ICST, 2009).
Oncescu, V., O’Dell, D. & Erickson, D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 13, 3232–3238 (2013).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Marriott, B. M. Food Components to Enhance Performance: An Evaluation of Potential Performance-Enhancing Food Components for Operational Rations (National Academic Press, 1994).
Robson, P. J. et al. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int. J. Sports Med. 20, 128–135 (1999).
Luger, A. et al. Acute hypothalamic–pituitary–adrenal responses to the stress of treadmill exercise. New Engl. J. Med. 316, 1309–1315 (1987).
Koc, S. The acute effect of aerobic exercise on serum cortisol levels of athletes and sedentary individuals. J. Educ. Train. Stud. 6, 29–36 (2018).
Hong, Y. J. et al. Multifunctional wearable system that integrates sweat-based sensing and vital-sign monitoring to estimate pre-/post-exercise glucose levels. Adv. Funct. Mater. 28, 1805754 (2018).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Sessler, D. I. Temperature monitoring and perioperative thermoregulation. Anesthesiology 109, 318–338 (2008).
Zhang, Y. et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296 (2019).
Yeung, C. et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics 13, 064125 (2019).
Lopez-Ramirez, M. A. et al. Built‐in active microneedle patch with enhanced autonomous drug delivery. Adv. Mater. 32, 1905740 (2020).
Webb, R. C. et al. Epidermal devices for noninvasive, precise and continuous mapping of macrovascular and microvascular blood flow. Sci. Adv. 1, e1500701 (2015).
Ma, Y. et al. Relation between blood pressure and pulse wave velocity for human arteries. Proc. Natl Acad. Sci. USA 115, 11144–11149 (2018).
Cho, H., Kim, H.-Y., Kang, J. Y. & Kim, T. S. How the capillary burst microvalve works. J. Colloid Interface Sci. 306, 379–385 (2007).
Choi, J. et al. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip 17, 2572–2580 (2018).
Acknowledgements
This work made use of the NUFAB facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), the MRSEC programme (NSF DMR-1720139) at the Materials Research Center, the International Institute for Nanotechnology (IIN), the Keck Foundation and the State of Illinois, through the IIN. J.U.K. and T.K. were supported by the Brain Research Program of the National Research Foundation (NRF) funded by the Korean government (MSIT; NRF-2019M3C7A1032076). J.C. acknowledges support from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; NRF-2019R1A2C1084419). J.A.R. acknowledge support from the National Institute on Aging of the National Institutes of Health (NIH R43AG067835). We thank the Querrey-Simpson Institute for Bioelectronics for support of this work.
Author information
Authors and Affiliations
Contributions
K.K., J.U.K. and J.A.R. conceived the idea, designed the research, analysed data and wrote the manuscript. K.K., J.U.K. and S.R.K. performed and were involved in the manufacturing of the sensors. K.K. designed the hardware for the wireless electronics platform. K.K., K.L. and I.Y. performed software design and software validation. Y.D. and Y.H. performed thermal and mechanical modelling. J.C., H.J., C.-J.S., Y.W., L.L., T.S.C., D.W. and J.-H.K. assisted with device fabrication. K.K. and J.U.K. performed research and led the experimental works with support from H.J., Y.P., T.K., R.G. and S.L.
Corresponding author
Ethics declarations
Competing interests
J.A.R., S.L. and R.G. are cofounders and/or employees of Epicore Biosystems, Inc., a company that pursues commercialization of microfluidic devices for wearable applications.
Additional information
Peer review information Nature Electronics thanks Tolga Kaya, Christopher Proctor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21, Tables 1–3 and notes 1–4.
Source data
Source Data Fig. 1
Source data for Fig. 1c (inset).
Source Data Fig. 2
Source data for Fig. 2c–i.
Source Data Fig. 4
Source data for Fig. 4c–i.
Source Data Fig. 5
Source data for Fig. 5d,e.
Rights and permissions
About this article
Cite this article
Kwon, K., Kim, J.U., Deng, Y. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nat Electron 4, 302–312 (2021). https://doi.org/10.1038/s41928-021-00556-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-021-00556-2
This article is cited by
-
Mussel-inspired resilient hydrogels with strong skin adhesion and high-sensitivity for wearable device
Nano Convergence (2024)
-
Towards on-skin analysis of sweat for managing disorders of substance abuse
Nature Biomedical Engineering (2024)
-
Radar near-field sensing using metasurface for biomedical applications
Communications Engineering (2024)
-
The challenges and promise of sweat sensing
Nature Biotechnology (2024)
-
A retrainable neuromorphic biosensor for on-chip learning and classification
Nature Electronics (2023)