Abstract
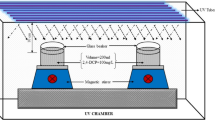
In this research work, we investigated the ability of the oxidative degradation of 2, 4-Dichlorophenoxy acetic acid herbicide via ultrasonic-assisted in electro-activation of the persulfate system in the presence of nano-zero valent iron. The effect of experimental parameters such as pH value [4–8], electrical current (0.5–1 A), persulfate concentration (0.25–0.5 mg.l−1), nano zero-valent iron dose (0.05–0.1 mg.l−1), and initial organic pollutant concentration (50–100 mg.l−1) on the ultrasonic-electropersulfate process performance was assessed via central composite design. The combination of ultrasonic waves with the electrochemical process to activation of persulfate showed better efficiency into 2, 4-Dichlorophenoxy acetic acid herbicide degradation compared to their implementation in individual and binary systems. Following optimal conditions (pH = 5.62, 0.80 A applied electrical current, 0.39 mg/L persulfate concentration, 0.07 mg/L nano-zero valent iron, and 50 mg/L 2,4-Dichlorophenoxy acetic acid concentration in 40 min reaction), nearly 91% removal was done. Moreover, the complete removal of 2, 4-Dichlorophenoxy acetic acid, 92% COD, and 88% TOC removal was achieved by this process near 140 min reaction. The scavenging experiment confirmed the role of free oxidizing species in the degradation of 2, 4-Dichlorophenoxy acetic acid during the process. Approximately 50% improved 2, 4-Dichlorophenoxy acetic acid removal in the process against the inclusive efficiency of single mechanisms. The obtained results were fitted to the pseudo-first-order kinetic model with a high correlation coefficient (R2 = 0.96). Five important intermediate products of 2, 4-D oxidation were 2, 4-dichlorophenol (2, 4-DCP), 2, 6-dichlorophenol (2, 6-DCP), 4, 6 dichlororesorcinol (4, 6-DCR), 2-chlorohydroquinone (2-CHQ), and 2-chloro-1, 4-benzoquinone (2-CBQ). In the end, can be employed as a satisfactory advanced oxidation process in high mineralization of 2, 4-D and refractory organic pollutants.










Similar content being viewed by others
References
Zand E, Baghestani MA, Soufizadeh S, PourAzar R, Veysi M, Bagherani N, et al. Broadleaved weed control in winter wheat (Triticum aestivum L.) with post-emergence herbicides in Iran. Crop Prot. 2007;26(5):746–52.
Kermani M, Mehralipour J, Kakavandi B. Photo-assisted electroperoxone of 2, 4-dichlorophenoxy acetic acid herbicide: kinetic, synergistic and optimization by response surface methodology. J Water Process Eng. 2019;32:100971.
Das R, Talat M, Srivastava O, Kayastha AM. Covalent immobilization of peanut β-amylase for producing industrial nano-biocatalysts: a comparative study of kinetics, stability and reusability of the immobilized enzyme. Food Chem. 2018;245:488–99.
Islam F, Wang J, Farooq MA, Khan MS, Xu L, Zhu J, et al. Potential impact of the herbicide 2, 4-dichlorophenoxyacetic acid on human and ecosystems. Environ Int. 2018;111:332–51.
Cotillas S, Sáez C, Cañizares P, Cretescu I, Rodrigo MA. Removal of 2, 4-D herbicide in soils using a combined process based on washing and adsorption electrochemically assisted. Sep Purif Technol. 2018;194:19–25.
Serbent MP, Rebelo AM, Pinheiro A, Giongo A, Tavares LBB. Biological agents for 2, 4-dichlorophenoxyacetic acid herbicide degradation. Appl Microbiol Biotechnol. 2019;103:5065–78.
Souza F, Saéz C, Lanza MR, Cañizares P, Rodrigo M. Removal of herbicide 2, 4-D using conductive-diamond sono-electrochemical oxidation. Sep Purif Technol. 2015;149:24–30.
Bradu C, Magureanu M, Parvulescu V. Degradation of the chlorophenoxyacetic herbicide 2, 4-D by plasma-ozonation system. J Hazard Mater. 2017;336:52–6.
Souza F, Sáez C, Lanza M, Cañizares P, Rodrigo M. Removal of chlorsulfuron and 2, 4-D from spiked soil using reversible electrokinetic adsorption barriers. Sep Purif Technol. 2017;178:147–53.
Kermani M, Shahsavani A, Ghaderi P, Kasaee P, Mehralipour J. Optimization of UV-Electroproxone procedure for treatment of landfill leachate: the study of energy consumption. J Environ Health Sci Eng. 2021:1–13. https://doi.org/10.1007/s40201-020-00583-9.
Cao M, Hou Y, Zhang E, Tu S, Xiong S. Ascorbic acid induced activation of persulfate for pentachlorophenol degradation. Chemosphere. 2019;229:200–5.
Khan NA, Khan SU, Ahmed S, Farooqi IH, Yousefi M, Mohammadi AA, et al. Recent trends in disposal and treatment technologies of emerging-pollutants-a critical review. TrAC Trends Anal Chem. 2020;122:115744.
Chi H, Wan J, Ma Y, Wang Y, Ding S, Li X. Ferrous metal-organic frameworks with stronger coordinatively unsaturated metal sites for persulfate activation to effectively degrade dibutyl phthalate in wastewater. J Hazard Mater. 2019;377:163–71.
Yegane Badi M, Vosoughi M, Sadeghi H, Mokhtari SA, Mehralipour J. Ultrasonic-assisted H2O2/TiO2 process in catechol degradation: kinetic, synergistic and optimisation via response surface methodology. Int J Environ Anal Chem. 2020:1–14. https://doi.org/10.1080/03067319.2020.1726335.
Yu J, Tang L, Pang Y, Zeng G, Wang J, Deng Y, et al. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: internal electron transfer mechanism. Chem Eng J. 2019;364:146–59.
Pedrosa M, Drazic G, Tavares PB, Figueiredo JL, Silva AM. Metal-free graphene-based catalytic membrane for degradation of organic contaminants by persulfate activation. Chem Eng J. 2019;369:223–32.
Wacławek S, Lutze HV, Grübel K, Padil VV, Černík M, Dionysiou DD. Chemistry of persulfates in water and wastewater treatment: a review. Chem Eng J. 2017;330:44–62.
Pu M, Ma Y, Wan J, Wang Y, Wang J, Brusseau ML. Activation performance and mechanism of a novel heterogeneous persulfate catalyst: metal–organic framework MIL-53 (Fe) with Fe II/Fe III mixed-valence coordinatively unsaturated iron center. Catal Sci Technol. 2017;7(5):1129–40.
Li X, Zhou M, Pan Y. Enhanced degradation of 2, 4-dichlorophenoxyacetic acid by pre-magnetization Fe-C activated persulfate: influential factors, mechanism and degradation pathway. J Hazard Mater. 2018;353:454–65.
Lv X-D, Yang S-Q, Xue W-J, Cui Y-H, Liu Z-Q. Performance of cu-cathode/Fe3+/peroxymonosulfate process on iohexol degradation. J Hazard Mater. 2019;366:250–8.
Si F, Zhang Y, Yao C, Du M, Hussain I, Huang S, et al. Degradation of ronidazole by electrochemically simultaneously generated persulfate and ferrous ions. Chemosphere. 2020;238:124579.
Dong H, Ning Q, Li L, Wang Y, Wang B, Zhang L, et al. A comparative study on the activation of persulfate by bare and surface-stabilized nanoscale zero-valent iron for the removal of sulfamethazine. Sep Purif Technol. 2020;230:115869.
Wang Q, Cao Y, Zeng H, Liang Y, Ma J, Lu X. Ultrasound-enhanced zero-valent copper activation of persulfate for the degradation of bisphenol AF. Chem Eng J. 2019;378:122143.
Yang L, Xue J, He L, Wu L, Ma Y, Chen H, et al. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: influences, mechanisms and prospective. Chem Eng J. 2019;378:122146.
Babu DJ, King P, Kumar YP. Optimization of cu (II) biosorption onto sea urchin test using response surface methodology and artificial neural networks. Int J Environ Sci Technol. 2019;16(4):1885–96.
Arasu MV, Arokiyaraj S, Viayaraghavan P, Kumar TSJ, Duraipandiyan V, Al-Dhabi NA, et al. One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J Photochem Photobiol B Biol. 2019;190:154–62.
Yousefi M, Nabizadeh R, Alimohammadi M, Mohammadi AA, Mahvi AH. Removal of phosphate from aqueous solutions using granular ferric hydroxide process optimization by response surface methodology. Desalin Water Treat. 2019;158:290–300.
Yousefi M, Arami SM, Takallo H, Hosseini M, Radfard M, Soleimani H, et al. Modification of pumice with HCl and NaOH enhancing its fluoride adsorption capacity: kinetic and isotherm studies. Hum Ecol Risk Assess Int J. 2019;25(6):1508–20.
Pan Y, Zhang Y, Zhou M, Cai J, Tian Y. Enhanced removal of emerging contaminants using persulfate activated by UV and pre-magnetized Fe0. Chem Eng J. 2019;361:908–18.
Moghaddam MH, Nabizadeh R, Dehghani MH, Akbarpour B, Azari A, Yousefi M. Performance investigation of Zeolitic Imidazolate framework–8 (ZIF-8) in the removal of trichloroethylene from aqueous solutions. Microchem J. 2019;150:104185.
Mazloomi S, Yousefi M, Nourmoradi H, Shams M. Evaluation of phosphate removal from aqueous solution using metal organic framework; isotherm, kinetic and thermodynamic study. J Environ Health Sci Eng. 2019;17(1):209–18.
Dehghani MH, Yetilmezsoy K, Salari M, Heidarinejad Z, Yousefi M, Sillanpää M. Adsorptive removal of cobalt (II) from aqueous solutions using multi-walled carbon nanotubes and γ-alumina as novel adsorbents: Modelling and optimization based on response surface methodology and artificial neural network. J Mol Liq. 2020;299:112154.
Pizarro C, Sáenz-González C, Pérez-del-Notario N, González-Sáiz JM. Development of an ultrasound-assisted emulsification–microextraction method for the determination of the main compounds causing cork taint in wines. J Chromatogr A. 2012;1229:63–71.
Esfe MH, Firouzi M, Rostamian H, Afrand M. Prediction and optimization of thermophysical properties of stabilized Al2O3/antifreeze nanofluids using response surface methodology. J Mol Liq. 2018;261:14–20.
Dehghani MH, Kamalian S, Shayeghi M, Yousefi M, Heidarinejad Z, Agarwal S, et al. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem J. 2019;145:486–91.
Dehghan A, Mohammadi AA, Yousefi M, Najafpoor AA, Shams M, Rezania S. Enhanced kinetic removal of ciprofloxacin onto metal-organic frameworks by sonication, process optimization and metal leaching study. Nanomaterials. 2019;9(10):1422.
Ayuba S, Mohammadib AA, Yousefic M, Changanic F. Performance evaluation of agro-based adsorbents for the removal of cadmium from wastewater. Desalin Water Treat. 2019;142:293–9.
Danmaliki GI, Saleh TA, Shamsuddeen AA. Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon. Chem Eng J. 2017;313:993–1003.
Arslan A, Topkaya E, Bingöl D, Veli S. Removal of anionic surfactant sodium dodecyl sulfate from aqueous solutions by O3/UV/H2O2 advanced oxidation process: process optimization with response surface methodology approach. Sustain Environ Res. 2018;28(2):65–71.
Iqbal M, Iqbal N, Bhatti IA, Ahmad N, Zahid M. Response surface methodology application in optimization of cadmium adsorption by shoe waste: a good option of waste mitigation by waste. Ecol Eng. 2016;88:265–75.
Fatah NAA, Triwahyono S, Jalil AA, Salamun N, Mamat CR, Majid ZA. n-Heptane isomerization over molybdenum supported on bicontinuous concentric lamellar silica KCC-1: Influence of phosphorus and optimization using response surface methodology (RSM). Chem Eng J. 2017;314:650–9.
Li Y-T, Li D, Lai L-J, Li Y-H. Remediation of petroleum hydrocarbon contaminated soil by using activated persulfate with ultrasound and ultrasound/Fe. Chemosphere. 2020;238:124657.
Pang Y, Ruan Y, Feng Y, Diao Z, Shih K, Hou L, et al. Ultrasound assisted zero valent iron corrosion for peroxymonosulfate activation for Rhodamine-B degradation. Chemosphere. 2019;228:412–7.
Lin H, Wu J, Zhang H. Degradation of bisphenol a in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep Purif Technol. 2013;117:18–23.
Bu L, Ding J, Zhu N, Kong M, Wu Y, Shi Z, et al. Unraveling different mechanisms of persulfate activation by graphite felt anode and cathode to destruct contaminants of emerging concern. Appl Catal B Environ. 2019;253:140–8.
Fogarasi S, Imre-Lucaci F, Fogarasi M, Imre-Lucaci Á. Technical and environmental assessment of selective recovery of tin and lead from waste solder alloy using direct anodic oxidation. J Clean Prod. 2019;213:872–83.
Wang X, Wang L, Li J, Qiu J, Cai C, Zhang H. Degradation of acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep Purif Technol. 2014;122:41–6.
Gao Y-q, Gao N-y, Wang W, S-f K, J-h X, H-m X, et al. Ultrasound-assisted heterogeneous activation of persulfate by nano zero-valent iron (nZVI) for the propranolol degradation in water. Ultrason Sonochem. 2018;49:33–40.
Miao D, Peng J, Wang M, Shao S, Wang L, Gao S. Removal of atorvastatin in water mediated by CuFe2O4 activated peroxymonosulfate. Chem Eng J. 2018;346:1–10.
Li X, Zhou M, Pan Y, Xu L. Pre-magnetized Fe0/persulfate for notably enhanced degradation and dechlorination of 2, 4-dichlorophenol. Chem Eng J. 2017;307:1092–104.
Huang K-C, Couttenye RA, Hoag GE. Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere. 2002;49(4):413–20.
Liang C, Wang Z-S, Bruell CJ. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere. 2007;66(1):106–13.
Ahmadi M, Ghanbari F. Combination of UVC-LEDs and ultrasound for peroxymonosulfate activation to degrade synthetic dye: influence of promotional and inhibitory agents and application for real wastewater. Environ Sci Pollut Res. 2018;25(6):6003–14.
Shen W, Wang Y, Zhan J, Wang B, Huang J, Deng S, et al. Kinetics and operational parameters for 1, 4-dioxane degradation by the photoelectro-peroxone process. Chem Eng J. 2017;310:249–58.
Fontmorin J-M, Huguet S, Fourcade F, Geneste F, Floner D, Amrane A. Electrochemical oxidation of 2, 4-dichlorophenoxyacetic acid: analysis of by-products and improvement of the biodegradability. Chem Eng J. 2012;195:208–17.
Chen H, Zhang Z, Yang Z, Yang Q, Li B, Bai Z. Heterogeneous Fenton-like catalytic degradation of 2, 4-dichlorophenoxyacetic acid in water with FeS. Chem Eng J. 2015;273:481–9.
Jaafarzadeh N, Ghanbari F, Ahmadi M. Catalytic degradation of 2, 4-dichlorophenoxyacetic acid (2, 4-D) by nano-Fe2O3 activated peroxymonosulfate: influential factors and mechanism determination. Chemosphere. 2017;169:568–76.
Cai J, Zhou M, Liu Y, Savall A, Serrano KG. Indirect electrochemical oxidation of 2, 4-dichlorophenoxyacetic acid using electrochemically-generated persulfate. Chemosphere. 2018;204:163–9.
Cai J, Zhou M, Yang W, Pan Y, Lu X, Serrano KG. Degradation and mechanism of 2, 4-dichlorophenoxyacetic acid (2, 4-D) by thermally activated persulfate oxidation. Chemosphere. 2018;212:784–93.
Acknowledgments
This work was supported by the Iran University of Medical Science [IUMS] (grant number: 96-04-212-31922).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehralipour, J., Kermani, M. Ultrasonic coupling with electrical current to effective activation of Persulfate for 2, 4 Dichlorophenoxyacetic acid herbicide degradation: modeling, synergistic effect, and a by-product study. J Environ Health Sci Engineer 19, 625–639 (2021). https://doi.org/10.1007/s40201-021-00633-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-021-00633-w