Abstract
This work was inspired by a previous report (Janjua et al. J. Phys. Chem. A 113, 3576–3587, 2009) in which the nonlinear-optical (NLO) response strikingly improved with an increase in the conjugation path. Herein, the effect of donor and acceptor substitutions on the geometrical parameters, electronic, optical and reactivity of argon in organic matrices has been computed by computational methods. The 6–311 ++ g(2d,2p) basis set and second order Moller–Plesset perturbation theory are used to explore mixed-argon benzonitrile compounds (1–7) namely; hydrogen argon cyanide, methyl argon cyanide, phenyl argon cyanide, cyano-phenyl argon cyanide, hydroxy-phenyl argon cyanide, nitro-phenyl argon cyanide and methoxy-phenyl argon cyanide respectively. Mullikan population analysis, natural bonding orbital (NBO) analysis, frontier molecular orbital analysis, molecular electrostatic potential surface analysis, polarizability and hyperpolarizability of noble gases in organic matrices have been studied. The results indicate that the electron donating groups (hydroxy, methoxy, phenyl and methyl) and electron withdrawing groups (nitro and cyano) fine tune the HOMO–LUMO orbitals and nonlinear optical properties. NBO analysis confirmed that these donor–acceptor groups support the charge transfer in our investigated jack-in-the-box compounds. The linear polarizability and first hyperpolarizability results suggest that all the studied compounds are good candidates for NLO response and associated applications.
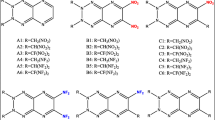
Graphic Abstract



Similar content being viewed by others
References
K. Ariga, J. P. Hill, M. V. Lee, A. Vinu, R. Charvet, and S. Acharya (2008). Sci Technol Adv Mater. https://doi.org/10.1088/1468-6996/9/1/014109.
G. V. Oshovsky, D. N. Reinhoudt, and W. Verboom (2007). Angew. Chem. Int. Ed. 46 (14), 2366–2393.
E. Fischer (1894). Ber. Dtsch. Chem. Grs. 27 (3), 3479–3483.
L. Khriachtchev, M. Räsänen, and R. B. Gerber (2009). Acc. Chem. Res. 42 (1), 183–191.
J. Kalinowski, R. B. Gerber, M. Räsänen, A. Lignell, and L. Khriachtchev (2014). J. Chem. Phys. 140 (9), 094303.
J.-M. Lehn (1993). Science 260 (5115), 1762–1764.
W. Luck and O. Schrems (1980). Spectrosc. Lett. 13 (10), 719–728.
A. Lignell, L. Khriachtchev, M. Pettersson, and M. Räsänen (2003). J. Chem. Phys. 118 (24), 11120–11128.
A. Lignell, L. Khriachtchev, H. Lignell, and M. Räsänen (2006). Phys. Chem. Chem. Phys. 8 (21), 2457–2463.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. J. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, D. 0109, Revision D. 01 (Gaussian Inc, Wallingford, 2009).
R. Dennington, T. Keith, and J. Millam, GaussView, version 5 (Semichem Inc., Shawnee Mission, 2009).
Ghiasuddin, M. Akram, M. Adeel, M. Khalid, M. N. Tahir, M. U. Khan, M. A. Asghar, M. A. Ullah, and M. Iqbal (2018). J. Mol. Struct. 1160, 129–141.
M. S. Ahmad, M. Khalid, M. A. Shaheen, M. N. Tahir, M. U. Khan, A. A. C. Braga, and H. A. Shad (2018). J. Phys. Chem. Solids 115, 265–276.
M. Shahid, M. Salim, M. Khalid, M. N. Tahir, M. U. Khan, and A. A. C. Braga (2018). J. Mol. Struct. 1161, 66–75.
R. Jawaria, M. Hussain, M. Khalid, M. U. Khan, M. N. Tahir, M. M. Naseer, A. A. C. Braga, and Z. Shafiq (2019). Synthesis. Inorg. Chim. Acta 486, 162–171.
M. Khalid, M. A. Ullah, M. Adeel, M. U. Khan, M. N. Tahir, and A. A. C. Braga (2019). J. Saudi Chem. Soc. 23 (5), 546–560.
M. N. Tahir, S. H. Mirza, M. Khalid, A. Ali, M. U. Khan, and A. A. C. Braga (2019). J. Mol. Struct. 1180, 119–126.
M. Haroon, M. Khalid, T. Akhtar, M. N. Tahir, M. U. Khan, M. Saleem, and R. Jawaria (2019). J. Mol. Struct. 1187, 164–171.
M. Rafiq, M. Khalid, M. N. Tahir, M. U. Ahmad, M. U. Khan, M. M. Naseer, A. A. C. Braga, and Z. Shafiq (2019). Appl. Organomet. Chem. 33, e5182.
B. Khan, M. Khalid, M. R. Shah, M. N. Tahir, M. U. Khan, A. Ali, and S. Muhammad (2019). Efficient Synthesis by Mono-Carboxy Methylation of 4, 4′-Biphenol. ChemistrySelect 4 (32), 9274–9284.
A. Hussain, M. U. Khan, M. Ibrahim, M. Khalid, A. Ali, S. Hussain, M. Saleem, N. Ahmad, S. Muhammad, and A. G. Al-Sehemi (2020). J. Mol. Struct. 1201, 127183.
M. Srnec and E. I. Solomon (2017). J. Am. Chem. Soc. 139 (6), 2396–2407.
R. Hussain, M. U. Khan, M. Y. Mehboob, M. Khalid, J. Iqbal, K. Ayub, M. Adnan, M. Ahmed, K. Atiq, and K. Mahmood (2020). ChemistrySelect 5 (17), 5022–5034.
R. A. Shehzad, J. Iqbal, M. U. Khan, R. Hussain, H. M. A. Javed, A. U. Rehman, M. U. Alvi, and M. Khalid (2020). Comp. Theor. Chem. 1181, 112833.
M. Y. Mehboob, R. Hussain, M. U. Khan, M. Adnan, A. Umar, M. U. Alvi, M. Ahmed, M. Khalid, J. Iqbal, and M. N. Akhtar (2020). Theor. Chem. 1186, 112908.
Z. Afzal, R. Hussain, M. U. Khan, M. Khalid, J. Iqbal, M. U. Alvi, M. Adnan, M. Ahmed, M. Y. Mehboob, and M. Hussain (2020). J. Mol. Model. 26 (6), 137–137.
G. Mahalakshmi and V. Balachandran (2015). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 135, 321–334.
M. R. S. A. Janjua (2012). Inorg. Chem. 51 (21), 11306–11314.
M. Mutailipu, Z. Xie, X. Su, M. Zhang, Y. Wang, Z. Yang, M. R. S. A. Janjua, and S. Pan (2017). J. Am. Chem. Soc. 139 (50), 18397–18405.
M. U. Khan, M. Khalid, M. Ibrahim, A. A. C. Braga, M. Safdar, A. A. Al-Saadi, and M. R. S. A. Janjua (2018). J. Phys. Chem. C 122 (7), 4009–4018.
M. R. S. A. Janjua, M. U. Khan, B. Bashir, M. A. Iqbal, Y. Song, S. A. R. Naqvi, and Z. A. Khan (2012). Comp. Theor. Chem. 994, 34–40.
M. R. S. A. Janjua, M. Amin, M. Ali, B. Bashir, M. U. Khan, M. A. Iqbal, W. Guan, L. Yan, and Z. M. Su (2012). Eur. J. Inorg. Chem. 2012 (4), 705–711.
M. U. Khan, M. Ibrahim, M. Khalid, M. S. Qureshi, T. Gulzar, K. M. Zia, A. A. Al-Saadi, and M. R. S. A. Janjua (2019). Chem. Phys. Lett. 715, 222–230.
M. U. Khan, M. Ibrahim, M. Khalid, A. A. C. Braga, S. Ahmed, and A. Sultan (2019). J. Cluster Sci. 30 (2), 415–430.
M. U. Khan, M. Ibrahim, M. Khalid, S. Jamil, A. A. Al-Saadi, and M. R. S. A. Janjua (2019). Chem. Phys. Lett. 719, 59–66.
M. R. S. A. Janjua (2021). Inorg. Chem. 60 (4), 2816–2828.
M. R. S. A. Janjua, R. Mahmood, M. Haroon, F. Anwar, M. U. Khan, and N. Ullah (2021). J Clust Sci. https://doi.org/10.1007/s10876-021-01997-7.
M. R. S. A. Janjua (2020). Chem Eur J. https://doi.org/10.1002/chem.202004299.
M. Haroon and M. R. S. A. Janjua (2020). Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2020.101880.
A. Karakas, A. Elmali, and H. Unver (2007). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 68 (3), 567–572.
Acknowledgements
The authors are thankful to the University of Sargodha, Pakistan and the Cornell University, USA for providing all the computational resources to complete this research article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sultana, N., Tariq, M.I., Siddique, U. et al. Theoretical Investigation of Jack-in-the-Box Electro-Optical Compounds: In-Silico Design of Mixed-Argon Benzonitriles Towards the Template of Clusters. J Clust Sci 33, 1185–1192 (2022). https://doi.org/10.1007/s10876-021-02052-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02052-1