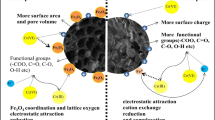
Abstract
Biochar (BC) has been extensively studied as adsorbent for the treatment of water pollution. Despite the distinct advantages, the high calcination temperature and low adsorption capacity of pristine BC limit its practical applications. Most of the former studies focused on the structure and/or surface modification to improve the adsorption capacity of BC. However, the harsh experiment conditions involved in the biochar modification limited the application in industrial level. Herein, we introduced mechanical treatment into BC preparation to reduce the calcination temperature and improve the adsorption capacity simultaneously. The results indicated that the calcination temperature was reduced and the adsorption capacity of the treated BC was improved after mechanochemical treatment. Characterization of the samples disclosed that BCs were graphitized with the particle size reduced to nanoscale after treatment. Adsorption tests indicated that the mechanochemically treated BCs showed much better removal performance of organic contaminants than that of pristine BCs. For instance, among four pristine BCs (BC600, BC700, BC800, and BC900), only BC900 has strong adsorption capacity for MB, while BC600 has low adsorption capacity (1.2 mg/g). By comparison, the adsorption capacity of MB increased greatly to 173.96 mg/g by BC600-500/1 (treated at 500 r/min for 1 hour). To optimize the mechanochemical treatment, the effects of rotation speed and agitation duration were also investigated.

Similar content being viewed by others
References
Ajmani A, Patra C, Subbiah S, Narayanasamy S (2020). Packed bed column studies of hexavalent chromium adsorption by zinc chloride activated carbon synthesized from Phanera vahlii fruit biomass. Journal of Environmental Chemical Engineering, 8(4): 103825
Ao W, Fu J, Mao X, Kang Q, Ran C, Liu Y, Zhang H, Gao Z, Li J, Liu G, Dai J (2018). Microwave assisted preparation of activated carbon from biomass: A review. Renewable & Sustainable Energy Reviews, 92:958–979
Bakshi S, Banik C, Rathke S J, Laird D A (2018). Arsenic sorption on zero-valent iron-biochar complexes. Water Research, 137: 153–163
Biswal B P, Chandra S, Kandambeth S, Lukose B, Heine T, Banerjee R (2013). Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. Journal of the American Chemical Society, 135(14): 5328–5331
Cheng B H, Zeng R J, Jiang H (2017). Recent developments of postmodification of biochar for electrochemical energy storage. Bioresource Technology, 246: 224–233
Choi J H, Lee C, Cho S, Moon G D, Kim B, Chang H, Jang H D (2018). High capacitance and energy density supercapacitor based on biomass-derived activated carbons with reduced graphene oxide binder. Carbon, 132: 16–24
Djellabi R, Zhao X, Bianchi C L, Su P D, Ali J, Yang B (2020). Visible light responsive photoactive polymer supported on carbonaceous biomass for photocatalytic water remediation. Journal of Cleaner Production, (269): 122286
Freundlich H M F (1906). Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie, 57A: 385–470
He L, Tong Z, Wang Z, Chen M, Huang N, Zhang W (2018). Effects of calcination temperature and heating rate on the photocatalytic properties of ZnO prepared by pyrolysis. Journal of Colloid and Interface Science, 509: 448–456
Ho Y S, McKay G (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70(2): 115–124
Hu R, Xiao J, Wang T, Chen G, Chen L, Tian X (2020). Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chemical Engineering Journal, (379): 122388
Huang Y, Xia S, Lyu J, Tang J (2019). Highly efficient removal of aqueous Hg2+ and CH3Hg+ by selective modification of biochar with 3-mercaptopropyltrimethoxysilane. Chemical Engineering Journal, 360: 1646–1655
Jonidi Jafari A, Kakavandi B, Jaafarzadeh N, Rezaei Kalantary R, Ahmadi M, Akbar Babaei A (2017). Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. Journal of Industrial and Engineering Chemistry, 45: 323–333
Julien P A, Užarević K, Katsenis A D, Kimber S A J, Wang T, Farha O K, Zhang Y, Casaban J, Germann L S, Etter M, Dinnebier R E, James S L, Halasz I, Friščić T (2016). In situ monitoring and mechanism of the mechanochemical formation of a microporous MOF-74 framework. Journal of the American Chemical Society, 138(9): 2929–2932
Kim J Y, Oh S, Park Y K (2020). Overview of biochar production from preservative-treated wood with detailed analysis of biochar characteristics, heavy metals behaviors, and their ecotoxicity. Journal of Hazardous Materials, 384: 121356
Kim Y, Oh J I, Vithanage M, Park Y K, Lee J, Kwon E E (2019). Modification of biochar properties using CO2. Chemical Engineering Journal, 372: 383–389
Kumar M, Xiong X, Wan Z, Sun Y, Tsang D C W, Gupta J, Gao B, Cao X, Tang J, Ok Y S (2020). Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresource Technology, (312): 123613
Lagergreen S (1898). Zur theorie der sogenannten adsorption gelöster stoffe kungliga svenska vetenskapsakademiens. Handlingar, 24(4): 1–39
Langmuir I (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40(9): 1361–1403
Li H, Mahyoub S A A, Liao W, Xia S, Zhao H, Guo M, Ma P (2017a). Effect of calcination temperature on characteristics and aromatic contaminants adsorption behavior of magnetic BC derived from calcination oil distillation residue. Bioresource Technology, 223: 20–26
Li W, Dang Q, Brown R C, Laird D, Wright M M (2017b). The impacts of biomass properties on calcination yields, economic and environmental performance of the calcination-bioenergy-BC platform to carbon negative energy. Bioresource Technology, 241: 959–968
Li Y, Su P, Li Y, Wen K, Bi G, Cox M (2018). Adsorption-desorption and degradation of insecticides clothianidin and thiamethoxam in agricultural soils. Chemosphere, 207: 708–714
Liu W J, Jiang H, Yu H Q (2015). Development of BC-based functional materials: toward a sustainable platform carbon material. Chemical Reviews, 115(22): 12251–12285
Liu Z, Adewuyi Y G, Shi S, Chen H, Li Y, Liu D, Liu Y (2019). Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chemical Engineering Journal, 366: 41–49
Lu L, Shan R, Shi Y, Wang S, Yuan H (2019). A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere, 222: 391–398
Lu Z, Li Y, Liu T, Wang G, Sun M, Jiang Y, He H, Wang Y, Zou P, Wang X, Zhao Q, Rao H (2020). A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chemical Engineering Journal, 389: 124417
Lyu H, Gao B, He F, Zimmerman A R, Ding C, Huang H, Tang J (2018). Effects of ball milling on the physicochemical and sorptive properties of biochar: experimental observations and governing mechanisms. Environmental Pollution, 233: 54–63
Mohan D, Kumar H, Sarswat A, Alexandre-Franco M, Pittman C U Jr (2014). Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chemical Engineering Journal, 236: 513–528
Peters J F, Iribarren D, Dufour J (2015). Biomass pyrolysis for biochar or energy applications? A life cycle assessment. Environmental Science & Technology, 49(8): 5195–5202
Qiu Y, Zheng Z, Zhou Z, Sheng G D (2009). Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresource Technology, 100(21): 5348–5351
Rajapaksha A U, Chen S S, Tsang D C W, Zhang M, Vithanage M, Mandal S, Gao B, Bolan N S, Ok Y S (2016). Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere, 148: 276–291
Sizmur T, Fresno T, Akgül G, Frost H, Moreno-Jiménez E (2017). Biochar modification to enhance sorption of inorganics from water. Bioresource Technology, 246: 34–47
Su P, Liu Y, Zhang J, Chen C, Yang B, Zhang C, Zhao X (2020a). Pbbased perovskite solar cells and the underlying pollution behind clean energy: dynamic leaching of toxic substances from discarded perovskite solar cells. Journal of Physical Chemistry Letters, 11(8): 2812–2817
Su P, Zhang J, Tang J, Zhang C (2019). Preparation of nitric acid modified powder activated carbon to remove trace amount of Ni(II) in aqueous solution. Water Science and Technology, 80(1): 86–97
Su P, Zhang J, Xiao K, Zhao S, Djellabi R, Li X, Yang B, Zhao X (2020b). C3N4 modified with single layer ZIF67 nanoparticles for efficient photocatalytic degradation of organic pollutants under visible light. Chinese Journal of Catalysis, 41(12): 1894–1905
Sun Y, Gao B, Yao Y, Fang J, Zhang M, Zhou Y, Chen H, Yang L (2014). Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chemical Engineering Journal, 240: 574–578
Talukdar K, Jun B M, Yoon Y, Kim Y, Fayyaz A, Park C M (2020). Novel Z-scheme Ag3PO4/Fe3O4-activated biochar photocatalyst with enhanced visible-light catalytic performance toward degradation of bisphenol A. Journal of Hazardous Materials, 398: 123025
Tang J, Liu Y, Su P, Quan J, Hu Y, Wang W, Zhang C (2020a). Removal of COD, NH4-N, and perfluorinated compounds from wastewater treatment plant effluent using ZnO-coated activated carbon. Water Science and Technology, 81(11): wst2020308
Tang J, Zhang C, Wang L, Hu Y, Su P, Wang W, He X (2020b). Photo-electrocatalytic degradation of cyclic volatile methyl siloxane by ZnO-coated aluminum anode: Optimal parameters, kinetics, and reaction pathways. Science of the Total Environment, 733: 139246
Teng C, Xie D, Wang J, Yang Z, Ren G, Zhu Y (2017). Ultrahigh conductive graphene paper based on ball-milling exfoliated graphene. Advanced Functional Materials, 27(20): 1700240
Tian Q, Wu W, Yang S, Liu J, Yao W, Ren F, Jiang C (2017). Zinc oxide coating effect for the dye removal and photocatalytic mechanisms of flower-like MoS2 nanoparticles. Nanoscale Research Letters, 12(1–10): 221
Tran H N, You S J, Hosseini-Bandegharaei A, Chao H P (2017). Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Research, 120: 88–116
Tsuzuki T, McCormick P G (2004). Mechanochemical synthesis of nanoparticles. Journal of Materials Science, 39(16–17): 5143–5146
Xiang W, Wan Y, Zhang X, Tan Z, Xia T, Zheng Y, Gao B. (2020). Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere, 255: 127057
Xiao J, Hu R, Chen G (2019). Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). Journal of Hazardous Materials, 387: 121980
Xu X, Zheng Y, Gao B, Cao X (2019). N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chemical Engineering Journal, 368: 564–572
Yang H, Hu Y, Zhang X, Qiu G (2004). Mechanochemical synthesis of cobalt oxide nanoparticles. Materials Letters, 58(3–4): 387–389
Yao Y, Lian C, Wu G, Hu Y, Wei F, Yu M, Wang S (2017). Synthesis of “sea urchin”-like carbon nanotubes/porous carbon superstructures derived from waste biomass for treatment of various contaminants. Applied Catalysis B: Environmental, 219: 563–571
Ye S, Yan M, Tan X, Liang J, Zeng G, Wu H, Song B, Zhou C, Yang Y, Wang H (2019). Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Applied Catalysis B: Environmental, 250: 78–88
Yu K L, Lau B F, Show P L, Ong H C, Ling T C, Chen W H, Ng E P, Chang J S (2017). Recent developments on algal biochar production and characterization. Bioresource Technology, 246: 2–11
Zhang C, Tan S, Niu X, Su P (2015). Treatment of geothermal water with high fluoride content by electrocoagulation. Desalination and Water Treatment, 54(8): 2223–2227
Zhang C, Wang K, Tan S, Niu X, Su P (2013). Evaluation and remediation of organics, nutrients and heavy metals in landfill leachate: A case study in Beijing. Chemistry and Ecology, 29(8): 668–675
Zhang J, Su P, Li Y, Li L (2020a). Environmental investigation of biomodification of steel slag through microbially induced carbonate precipitation. Journal of Environmental Sciences-China, 101: 282–292
Zhang J, Zhao H, Li J, Jin H, Yu X, Lei Y, Wang S (2019). In situ encapsulation of iron complex nanoparticles into biomass-derived heteroatom-enriched carbon nanotubes for high-performance super-capacitors. Advanced Energy Materials, 9(4): 1803221
Zhang S, Jiang S F, Huang B C, Shen X C, Chen W J, Zhou T P, Cheng H Y, Cheng B H, Wu C Z, Li W W, Jiang H, Yu H Q (2020b). Sustainable production of value-added carbon nanomaterials from biomass calcination. Nature Sustainability, 2020: 1–8
Zhang Y, Lu L, Zhang S, Lv Z, Yang D, Liu J, Chen Y, Tian X, Jin H, Song W (2018). Biomass chitosan derived cobalt/nitrogen doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 6(14): 5740–5745
Zhang Y, Su P, Weathersby D, Zhang Q, Zheng J, Fan R, Dai Q (2020c). Synthesis of γ-Fe2O3-ZnO-biochar nanocomposites for Rhodamine B removal. Applied Surface Science, 501: 144217: 1–7
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21777106), the Natural Science Foundation of Guangdong Province, China (No. 2017A030313046) and Basic Research Project of Shenzhen City, China (No. JCYJ20170818093429961).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Mechanochemical treatment reduced the calcination temperature for biochar synthesis.
• Biochar is converted to graphite after mechanochemical treatment.
• Biochar was reduced to nanoscale after mechanochemical treatment.
Credit Author Statement
Peidong Su: Methodology, experimental operation, original draft preparation.
Xiangyu Gao: Manuscript revise, English polish, and result discussion.
Junke Zhang: Adsorption kinetic analysis and sorption isotherms, calculation.
Ridha Djellabi: Language checking.
Bo Yang: Supervision, writing- reviewing and editing.
Qi Wu: Characterization, data analysis.
Zhen Wen: XPS analysis.
Supporting Information
Rights and permissions
About this article
Cite this article
Su, P., Gao, X., Zhang, J. et al. Enhancing the adsorption function of biochar by mechanochemical graphitization for organic pollutant removal. Front. Environ. Sci. Eng. 15, 130 (2021). https://doi.org/10.1007/s11783-021-1418-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-021-1418-2