Abstract
Introduction
The success of parallel Magnetic Resonance Imaging algorithms like SENSitivity Encoding (SENSE) depends on an accurate estimation of the receiver coil sensitivity maps. Deep learning-based receiver coil sensitivity map estimation depends upon the size of training dataset and generalization capabilities of the trained neural network. When there is a mismatch between the training and testing datasets, retraining of the neural networks is required from a scratch which is costly and time consuming.
Materials and methods
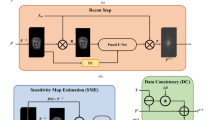
A transfer learning approach, i.e., end-to-end fine-tuning is proposed to address the data scarcity and generalization problems of deep learning-based receiver coil sensitivity map estimation. First, generalization capabilities of a pre-trained U-Net (initially trained on 1.5T receiver coil sensitivity maps) are thoroughly assessed for 3T receiver coil sensitivity map estimation. Later, end-to-end fine-tuning is performed on the pre-trained U-Net to estimate the 3T receiver coil sensitivity maps.
Result and conclusion
Peak Signal-to-Noise Ratio, Root Mean Square Error and central line profiles (of the SENSE reconstructed images) show a successful SENSE reconstruction by utilizing the receiver coil sensitivity maps estimated by the proposed method.






Similar content being viewed by others
References
McRobbie DW, Moore EA, Graves MJ (2017) MRI from picture to proton. MRI Pict Proton. https://doi.org/10.2214/ajr.182.3.1820592
Hamilton J, Franson D, Seiberlich N (2017) Recent advances in parallel imaging for MRI. Prog Nucl Magn Reson Spectrosc 101:71–95
Siddiqui MF, Reza AW, Shafique A, Omer H, Kanesan J (2017) FPGA implementation of real-time SENSE reconstruction using pre-scan and Emaps sensitivities. Magn Reson Imaging 44:82–91
Irfan AS, Nisar A, Shahzad H, Omer H (2016) Sensitivity maps estimation using eigenvalues in sense reconstruction. Appl Magn Reson 47:487–498
Adalsteinsson E, Aksoy M, Atkinson D, Bammer R, Batchelor P, Benner T, Bock M, Casselman J, Dietrich O, Duensing R, Eibel R, Essig M, Fink C, Finn J, Fischer B, Griswold M, Herrmann K, Hoogeveen R, Huber A, Zech C (2007) Parallel imaging in clinical MR applications. Springer, Berlin. https://doi.org/10.1007/978-3-540-68879-2
Biyik E, Ilicak E, Cukur T (2018) Reconstruction by calibration over tensors for multi-coil multi-acquisition balanced SSFP imaging. Magn Reson Med 79:2542–2554
Liu F, Samsonov A, Chen L, Kijowski R, Feng L (2019) SANTIS: sampling-augmented neural network with incoherent structure for MR image reconstruction. Magn Reson Med 82:1890–1904
Dar SUH, Özbey M, Çatlı AB, Çukur T (2020) A transfer-learning approach for accelerated mri using deep neural networks. Magn Reson Med 84:663–685
Han Y, Yoo J, Kim HH, Shin HJ, Sung K, Ye JC (2018) Deep learning with domain adaptation for accelerated projection-reconstruction MR. Magn Reson Med 80:1189–1205
Lee D, Yoo J, Ye JC (2017) Deep artifact learning for compressed sensing and parallel MRI. arXiv preprint arXiv:1703.01120
Hyun CM, Kim HP, Lee SM, Lee S, Seo JK (2018) Deep learning for undersampled MRI reconstruction. Phys Med Biol 63:135007
Gözcü B, Mahabadi RK, Li Y-H, Ilıcak E, Cukur T, Scarlett J, Cevher V (2018) Learning-based compressive MRI. IEEE Trans Med Imaging 37:1394–1406
Peng X, Perkins K, Cli B, Sutton B, Liang Z (2018) Deep-SENSE: learning coil sensitivity functions for SENSE reconstruction using deep learning. In: Joint Annual Meeting ISMRM-ESMRMB, 2018. https://archive.ismrm.org/2018/3528.html
Chen Z, Chen Y, Li D, Christodoulou AG (2019) A deep learning algorithm for non-cartesian coil sensitivity map estimation. In: ISMRM 27th Annual meeting and exhibition. https://archive.ismrm.org/2019/4783.html
Meng N, Yang Y, Xu Z, Sun J (2019) A Prior learning network for joint image and sensitivity estimation in parallel MR imaging BT-medical image computing and computer assisted intervention–MICCAI 2019 . In: Shen D, Liu T, Peters TM, Staib LH, Essert C, Zhou S, Yap P-T, Khan A (eds) International conference on medical image computing and computer-assisted intervention. Springer International Publishing, Cham, pp 732–740
Dar SUH, Yurt M, Shahdloo M, Ildız ME, Tınaz B, Çukur T (2020) Prior-guided image reconstruction for accelerated multi-contrast MRI via generative adversarial networks. IEEE J Sel Top Signal Process 14:1072–1087
Tajbakhsh N, Shin JY, Gurudu SR, Hurst RT, Kendall CB, Gotway MB, Liang J (2016) Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging 35:1299–1312
Knoll F, Hammernik K, Kobler E, Pock T, Recht MP, Sodickson DK (2019) Assessment of the generalization of learned image reconstruction and the potential for transfer learning. Magn Reson Med 81:116–128
Hendrick RE (2008) Breast MRI: fundamentals and technical aspects. Breast MRI Fundam Tech Asp. https://doi.org/10.1007/978-0-387-73507-8
Wiesinger F, Van De Moortele PF, Adriany G, De Zanche N, Ugurbil K, Pruessmann KP (2004) Parallel imaging performance as a function of field strength: an experimental investigation using electrodynamic scaling. Magn Reson Med 52:953–964
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells WM, Frangi AF (eds) Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015. Springer International Publishing, Cham, pp 234–241
Bahadir CD, Dalca AV, Sabuncu MR (2019) Learning-Based Optimization of the Under-Sampling Pattern in MRI. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics). Springer, Cham, pp 780–792
Kingma DP, Ba JL (2015) Adam: a method for stochastic optimization. In: 3rd International Conference on Learning Representations-ICLR 2015-Conference Track Processdings, pp 1–15
Chollet F & others (2015) Keras. GitHub. Retrieved from https://github.com/fchollet/keras
Kramer O (2016) Scikit-learn. Machine learning for evolution strategies. Springer, Cham, pp 45–53
Guerquin-Kern M (2012) Matlab code for MRI simulation and reconstruction. Department of Computer Science Engineering University, Calcutta
Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL (2007) Open access series of imaging studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci 19:1498–1507
Chow LS, Rajagopal H, Paramesran R (2016) Correlation between subjective and objective assessment of magnetic resonance (MR) images. Magn Reson Imaging 34:820–831
Wood R, Bassett K, FoersterSpryTong VCL (2012) 1.5 tesla magnetic resonance imaging scanners compared with 3.0 tesla magnetic resonance imaging scanners: systematic review of clinical effectiveness. CADTH Technol Overv 2:e2201–e2201
Pruessmann KP (2004) Parallel imaging at high field strength: synergies and joint potential. Top Magn Reson Imaging 15:237–244
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Madiha Arshad declares that she has no conflict of interest. Mahmood Qureshi declares that he has no conflict of interest. Omair Inam declares that he has no conflict of interest. Hammad Omer declares that he has no conflict of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arshad, M., Qureshi, M., Inam, O. et al. Transfer learning in deep neural network-based receiver coil sensitivity map estimation. Magn Reson Mater Phy 34, 717–728 (2021). https://doi.org/10.1007/s10334-021-00919-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00919-y