Abstract
Resurgence is an increase in a previously reinforced behavior following a worsening of conditions for a more recently reinforced behavior. Discrimination training is incorporated into treatment for problem behavior to prevent treatment adherence failures that may result in resurgence. There is evidence that resurgence may be reduced when a stimulus that signals alternative-response extinction is present compared with absent; however, the generality of this effect is unknown given the limited testing conditions. The goal of the present experiments was to further examine the effects of such stimuli in a reverse-translational evaluation using rats. Target responding was reinforced in baseline and then placed on extinction in the following discrimination-training phase. An alternative response was differentially reinforced in a two-component multiple schedule where one stimulus (i.e., SD) signaled alternative-response reinforcement and the other (i.e., SΔ) signaled extinction. Experiment 1 assessed resurgence in both the SΔ and SD when alternative reinforcement was removed. Experiment 2 evaluated resurgence under conditions that better approximated those used in the clinic in which the alternative-response SΔ was present or absent. The SΔ failed to suppress target responding during resurgence testing in both experiments. These findings suggest that the conditions under which an alternative-response SΔ will successfully mitigate resurgence may be limited and require further research.
Similar content being viewed by others
Individuals with neurodevelopmental disorders may engage in severe forms of problem behavior (Emerson et al., 2001; Harvey et al., 2009), and such behavior may threaten the safety and well-being of the individual and their caregivers (e.g., Iwata et al., 1994). The most commonly used and empirically validated treatment for problem behavior in this population is functional communication training (FCT; Durand & Moskowitz, 2015; Greer et al., 2018; Kurtz et al., 2011; Tiger et al., 2008). During FCT, the problem behavior is placed on extinction, and the client is taught an alternative functional communicative response (FCR) to request the reinforcer that had previously maintained the problem behavior. As a result, problem behavior decreases and rates of the FCR increase (e.g., Carr & Durand, 1985).
Despite the efficacy of FCT for reducing problem behavior, such behavior is susceptible to resurgence. Resurgence refers to the increase of a previously reinforced behavior following a worsening of conditions for a more recently reinforced alternative behavior (Epstein, 1985; Lattal & Wacker, 2015; Shahan & Craig, 2017). Resurgence of problem behavior may occur if the conditions of FCR reinforcement are worsened in some way. For example, resurgence of problem behavior has been observed following FCR extinction (e.g., Volkert et al., 2009), temporary failures in treatment adherence (e.g., Marsteller & St Peter, 2012; St. Peter Pipkin et al., 2010), and reductions in FCR reinforcement rate (e.g., Briggs et al., 2018). Thus, resurgence poses a serious concern for maintaining positive treatment effects. Importantly, research on resurgence is critical to the development of more effective treatment approaches for problem behaviors in clinical populations (Greer & Shahan, 2019; St. Peter, 2015).
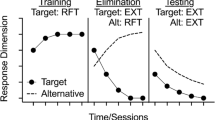
In addition to evaluating resurgence during FCT in clinical settings, resurgence may be examined in the basic laboratory with nonhuman subjects such as rats or pigeons. Given the procedural similarities between those used in treatment and those used in the laboratory, such research has direct implications for the development of effective clinical treatments. In the laboratory, resurgence may be studied using a three-phase procedure. First, a target response is reinforced in baseline (e.g., pressing the right lever). Second, the target response is placed on extinction while reinforcement is made available for an alternative behavior (e.g., pressing the left lever) in the treatment phase. Finally, resurgence of target responding may be assessed by placing the alternative response on extinction. Resurgence is evident if rates of the target response increase in the final phase relative to those at the end of the second phase (e.g., Craig & Shahan, 2016; Shahan et al., 2020; Winterbauer & Bouton, 2010). Given the clinical significance of resurgence, a considerable amount of research using both human participants and nonhuman subjects has been conducted to identify variables that may be used to mitigate this effect (Wathen & Podlesnik, 2018).
Several basic laboratory experiments have shown that alternating periods of alternative-response reinforcement with extinction during treatment reduces resurgence compared with treatment with constant alternative reinforcement (Schepers & Bouton, 2015; Shahan et al., 2020; Thrailkill et al., 2019; Trask et al., 2018; but see Sweeney & Shahan, 2013b). For example, Shahan et al. (2020) compared the effects of such on/off alternative reinforcement to constant alternative reinforcement available for a range of treatment durations. Resurgence systematically decreased across successive alternative-response extinction sessions and was significantly smaller compared with resurgence following all five durations of constant alternative reinforcement. To explain this effect, Shahan et al. (2020) expanded on previous arguments (i.e., Trask et al., 2018) that on/off alternative-reinforcement results in smaller resurgence effects as the result of improved discrimination of the current response-reinforcer contingencies signaled by the presence and absence of alternative reinforcers. Importantly, this conclusion suggests that improved discrimination of alternative response-reinforcer contingencies may be a promising variable in mitigating resurgence.
Discrimination of response-reinforcer contingencies is traditionally established through discrimination training. In discrimination training, a particular response is reinforced only in the presence of an SD stimulus, and that response is extinguished in the presence of the SΔ stimulus (e.g., Rilling, 1977). That is, the SD signals that reinforcement is available for a particular response while the SΔ signals that reinforcement is not available. Effective discrimination is evident by differential responding in the presence of these stimuli (e.g., Balsam, 1988), such that the behavior may occur more frequently in the presence of the SD stimulus and less frequently in the presence of the SΔ stimulus.
Importantly, there is evidence that stimuli paired with reinforcement or extinction may mitigate resurgence and other forms of behavioral relapse. For example, Craig, Browning, and Shahan (2017) observed reduced resurgence of lever pressing in rats when a discrete visual stimulus previously paired with target and alternative reinforcement (i.e., the light in the food aperture) was presented response dependently when the alternative response was placed on extinction during testing. Similarly, Trask (2019) found that presentation of a tone (response dependently or response independently) previously paired with both target–response extinction and alternative reinforcement mitigated resurgence of target responding in rats. Additionally, presentation of discrete stimuli associated with target-response extinction has also been shown to reduce other forms of relapse in rats, including reinstatement, spontaneous recovery (Bernal-Gamboa et al., 2017), and renewal (Nieto et al., 2017; Willcocks & McNally, 2014). However, a clear gap in this literature is evaluation of a stimulus paired with alternative-response extinction.
Alternative-response discrimination training is often incorporated into FCT in the clinic in order to increase the feasibility of treatment implementation and to reduce the risk of failures in treatment adherence. That is, the FCR is typically reinforced according to a dense schedule of reinforcement to facilitate response acquisition early in treatment; however, this may result in unmanageably high rates of responding. If the FCR occurs at a rate too high for the caregivers or therapists to consistently reinforce, the FCR may contact extinction resulting in resurgence. To reduce this possibility, discriminative stimuli may be used to differentially signal when FCR reinforcement is or is not available. This discrimination is typically established during FCT by use of a two-component multiple schedule in which the FCR is reinforced in one component signaled by the SD and the FCR is extinguished in the other component signaled by the SΔ, while problem behavior is placed on extinction in both components (Saini et al., 2016). The duration of the SΔ component may also be increased to reduce the overall rate of FCR reinforcement to further control the rate of the FCR (e.g., Betz et al., 2013; Hanley et al., 2001).
Expanding on the discrete stimuli and relapse literature reviewed above, there is evidence to suggest that FCR discrimination training may be used to reduce resurgence. Two studies have demonstrated significant reductions in resurgence of destructive behavior in the presence of the FCR SΔ stimulus following FCR discrimination training. The first study, Fuhrman et al. (2016) assessed resurgence of destructive behavior in two children following FCT with and without FCR discrimination training. During discrimination training, the FCR was reinforced in the SD component (signaled by a green index card) and not in the SΔ component (signaled by a red index card) of a two-component multiple schedule, and problem behavior was placed on extinction in both components. Resurgence was then tested during extended exposure to the SΔ stimulus in which reinforcement for the FCR was never available. In the control condition, traditional FCT was conducted without discrimination training in which problem behavior was placed on extinction while the FCR was reinforced. Following this traditional FCT, resurgence was tested by placing the FCR on extinction and no discriminative stimuli were presented. Resurgence of destructive behavior was substantially reduced in the SΔ condition following FCT with discrimination training compared with resurgence following traditional FCT. These findings suggest that presentation of a stimulus that signals extinction of the alternative response may reduce or prevent resurgence of the target behavior.
It is important to note that one limitation of this study makes interpretation of their findings difficult. That is, the obtained rate of reinforcement for the FCR was lower in the discrimination FCT treatment condition compared with the traditional FCT condition. While Fuhrman et al. (2016) intentionally thinned the rate of reinforcement for the FCR by increasing the duration of the SΔ component relative to the SD component during discrimination training, there is substantial evidence to suggest that lower rates of alternative reinforcement generate less resurgence than higher rates when removed (Bouton & Trask, 2016; Craig et al., 2016; Craig & Shahan, 2016; Sweeney & Shahan, 2013a).
In a follow-up study, Fisher et al. (2020) extended these findings and addressed this limitation. Prior to treatment proper, FCR discrimination was trained using the multiple-schedule FCT procedure. That is, the FCR was reinforced in the SD component but not in the SΔ component, and the rate of FCR reinforcement was reduced by increasing the duration of the SΔ component. Following this pretraining, FCT was evaluated in two separate contexts using a multielement design. The overall FCT procedures were identical across contexts such that problem behavior was reinforced during baseline, problem behavior was extinguished while the FCR was reinforced during treatment, and resurgence was tested by placing the FCR on extinction. The contexts differed by the presence or absence of the discriminative stimuli previously established in pretraining. In the first context, only the SD stimulus was presented during the treatment phase, and only the SΔ stimulus was presented during resurgence testing. In the second context, the discriminative stimuli were not present during treatment or testing. Importantly, the researchers controlled for the rate of FCR reinforcement across contexts. Consistent with the findings of from the initial study, resurgence of destructive behavior was substantially reduced in the presence of the SΔ stimulus compared with in its absence. Because Fisher et al. (2020) controlled for the rate of alternative reinforcement across conditions, this experiment provides more compelling evidence that resurgence may be prevented by a stimulus that signals extinction of the alternative response.
However, given the limited testing conditions, the generality of these findings is unknown. That is, Fuhrman et al. (2016) and Fisher et al. (2020) assessed resurgence only in the presence and absence of the alternative-response SΔ and did not include tests under SD conditions. Thus, it is not surprising that resurgence did not occur under conditions in which the alternative response was never reinforced. To extend these findings, the procedures reported in Fuhrman et al. (2016) and Fisher et al. (2020) were approximated in two reverse-translational experiments. The purpose of Experiment 1 was to determine whether resurgence would be mitigated in the SΔ if the alternative response contacts extinction under conditions in which it was previously reinforced (i.e., the SD). The purpose of Experiment 2 was to evaluate resurgence in the presence of the SD alone, the SΔ alone, and in the absence of discriminative stimuli altogether and to compare these to the multiple-schedule test condition of Experiment 1.
Experiment 1
The purpose of Experiment 1 was to determine whether resurgence would be mitigated in the presence of the alternative-response SΔ stimulus if the alternative response is also placed on extinction in the presence of the SD stimulus that had previously signaled availability of alternative reinforcement. The general procedures reported by Fuhrman et al., (2016) and Fisher et al. (2020) were approximated in a reverse-translational experiment with rats as subjects. Following baseline in which target lever pressing was reinforced, rats received discrimination training in which alternative lever pressing was reinforced in one component of a multiple schedule signaled by the SD stimulus and extinguished in the second component signaled by the SΔ stimulus, while target lever pressing was placed on extinction in both. In the final phase, resurgence of target responding was examined in the presence of both SΔ and SD when the alternative response was also placed on extinction in the SD component.
Method
Subjects
Five experimentally naïve male Long-Evans rats served as subjects. Rats were approximately 71–90 days old upon arrival and were individually housed in a temperature and humidity controlled colony room with a 12:12/hr light–dark cycle (lights on at 7:00 a.m.). Throughout the experiment rats had ad libitum access to water in the home cages and were maintained at 80% of their free-feeding weights by supplemental postsession feeding. All experimental procedures described below were conducted in accordance with Utah State University’s Institutional Animal Review Committee guidelines.
Apparatus
Five identical Med Associates (St. Albans, VT) operant chambers were used. Chambers measured 30 cm × 24 cm × 21 cm and were housed in sound and light attenuating cubicles. Each chamber was constructed of two aluminum side panels, and a clear Plexiglas ceiling, door, and back wall. Two retractable levers on the right-side panel, with stimulus lights above them, were positioned on either side of a food receptacle that was illuminated when 45-mg grain-based food pellets (Bio Serv, Flemington, NJ) were delivered. A house light positioned at the top center of the left-side panel was used for general chamber illumination. A 2900 Hz tone generator positioned to the right of the house light was used to emit a 65-dB tone. A white noise generator positioned adjacent to the chamber cubicles was used to emit white noise and mask extraneous sound during each experimental session. All experimental events and data collection were controlled by Med-PC software run on a computer in an adjacent control room.
Procedure
Experimental sessions were conducted seven days per week during the light cycle at approximately the same time each day. All sessions were at least 30 min, excluding time for reinforcement delivery with the exception that session time during the discrimination training and test phases could exceed 30 min (see below). During reinforcement deliveries, all experimental timers were paused for 4 s, the pellet dispenser dropped a single food pellet into the illuminated food receptacle, the lever stimulus lights darkened, and, when applicable, the discriminative stimuli remained present.
Training
Rats were first trained to consume pellets from the lit food aperture for three 30-min sessions. Food pellets were delivered response independently according to a variable time (VT) 60-s schedule, such that a single food pellet was delivered, on average, every 60 s. The VT schedule and all variable-interval (VI) schedules described below consisted of 10 intervals derived from Fleshler and Hoffman’s (1962) constant-probability distribution. Levers remained retracted and lever-stimulus and house lights were darkened throughout training.
Phase 1: Baseline
Sessions during baseline began with insertion of the target lever (right–left, counterbalanced across rats) and illumination of the target-lever stimulus light. To facilitate target-response acquisition, the first response in the first Baseline session immediately produced a food pellet (i.e., fixed-ratio 1 schedule). Thereafter, target responding was reinforced according to a VI 30-s schedule, such that a single food pellet was delivered following the target response, on average, every 30 s, for the remainder of the session and phase. This phase lasted 20 sessions.
Phase 2: Target extinction + alternative discrimination training
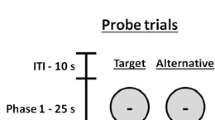
Sessions during this phase began with insertion of both the target and alternative levers and illumination of both lever stimulus lights. A two-component multiple schedule, comprised of an SD and an SΔ component, was used to train alternative-response discrimination. Components were signaled by either a constant house light and tone or flashing house light and pulsing tone (on/off every 0.5 s), counterbalanced across rats. Responses to the alternative lever were reinforced according to a VI 5 s schedule in the SD component and were placed on extinction in the SΔ component. Target responding was extinguished in both components. The VI timer only counted down during the SD component, and if the VI timer did not elapse before the end of the SD component, it was paused until the next SD presentation, thereafter the timer continued. Additionally, if the VI timer elapsed and the rat did not earn the food pellet before the end of the SD component, the food could not be earned until the next SD component began. Each component was presented 15 times in strict alternation for a total of 30 component presentations per session, and component durations ranged from 10 to 110 s, averaging 1 min (components were programed in this manner based on previous research from our lab, see Shahan, 2002). In the first session, the SD component was presented first, and the first alternative lever press immediately produced a food pellet, after which the VI timer began and components strictly alternated. During all subsequent sessions, the first component was selected with p = .50. A 3-s change over delay (COD) in the SΔ component was arranged such that any alternative response made in the final 3 s of the SΔ component delayed transition to the SD component until an alternative response was not made for 3 s. The COD was included to avoid adventitious reinforcement of alternative responding in the SΔ component by transition to the SD component. Thus, time in SΔ could exceed time in SD, depending on individual rat’s performance. This phase lasted 25 sessions.
Phase 3: Test
Sessions during the Test phase were identical to those in the previous phase, with the exception that the alternative response was no longer reinforced in the SD component. Thus, target and alternative behaviors were assessed during extinction during both the SD and SΔ components. This phase lasted five sessions.
Data analyses
The primary dependent variables were target and alternative responses per min across sessions and between components. Additionally, a discrimination index (DI) was calculated to evaluate alternative response discrimination in each session of Phases 2 and 3 by dividing alternative responses in the SD component by total alternative responses in the SD and SΔ components. For all analyses, the within-subject factors were session and component, and there was no between-subjects factor. Statistical significance was determined using α = .05 and a Bonferroni adjustment was applied to all simple effects follow-up analyses.
Results
Table 1 provides a summary of mean response rates, discrimination indices, and reinforcer rates across phases.
Phase 1: Baseline
Target response rates increased across sessions of Phase 1 for all rats to an average of 24.21 responses per min (see Table 1).
Phase 2: Target extinction + alternative discrimination training
The top panel of Fig. 1 displays mean alternative response rates in the SD and SΔ components across sessions of Phase 2. A 2 × 25 (Component × Session) repeated-measures ANOVA conducted on these data revealed significant effects of Component, F(1, 4) = 18.93, p = .01, ηp2 = .83, and Session, F(24, 96) = 8.71, p < .001, ηp2 = .69, and a significant Component × Session interaction, F(24, 96) = 8.06, p < .001, ηp2 = .67. A simple effects test conducted following the significant interaction revealed that alternative responding did not differ between SD and SΔ components in the first session of Phase 2 (p = .21) but responding was higher in the SD relative to the SΔ component in the final session (p = .008). These results suggest that alternative responding increased across sessions in the SD component and remained relatively low in the SΔ component.
Mean alternative (top panel) and target (bottom panel) responses per min in the SD and SΔ components across sessions of Phase 2 in Experiment 1. Error bars represent standard error of the mean
Table 1 displays the average alternative-response DI from the last session of Phase 2. A DI greater than 0.50 indicates that responding was proportionally higher in the SD component than in the SΔ component. A one-sample t test conducted on these data against the test value of 0.50 suggested that the proportion of responding in the SD was significantly greater than 0.50, t(4) = 5.45, p = .003, d = 2.43. Taken together, the data in the top panel of Fig. 1 and the alternative-response DIs suggest that rats effectively allocated alternative responding according to the arranged discriminative stimuli.
The bottom panel of Fig. 1 displays mean target response rates in the SD and SΔ components across sessions of Phase 2. A 2 × 25 (Component × Session) repeated-measures ANOVA conducted on these data revealed significant effects of Component, F(1, 4) = 18.98, p = .01, ηp2 = .83, and Session, F(24, 96) = 14.66, p < .001, ηp2 = .79, and a nonsignificant Component × Session interaction, F(24, 96) = 1.33, p = .17, ηp2 = .25. Simple effect tests conducted following the significant effects of Session and Component revealed that target responding was lower in the final session of this phase relative to the first (p = .002), regardless of component, and was overall higher in the SΔ compared with the SD component (p = .01), regardless of session. Thus, target responding decreased across sessions of Phase 2 in both components but remained generally elevated in the SΔ component (see Table 1 for Phase 2 terminal target response rates in both components).
Phase 3: Test
Figure 2 displays mean target response rates during the last session of Phase 2 and the first session of Phase 3 in the SD and SΔ components. A 2 × 2 (Component × Session) repeated-measures ANOVA conducted on these data revealed a significant effect of Component, F(1, 4) = 14.76, p = .012, ηp2 = .79, and Session, F(1, 4) = 18.09, p = .01, ηp2 = .82, and a nonsignificant Component × Session interaction, F(1, 4) = 0.37, p = .58, ηp2 = .08. Simple effects tests conducted following the significant effects of Session and Component revealed that target responding increased between the last session of Phase 2 and the first session of Phase 3 (p = .01), regardless of component, and that responding was overall higher in the SΔ compared with the SD component (p = .02), regardless of session. Thus, resurgence was evident in both components but the overall level of responding differed between components.
Mean target responses per min in the last session of Phase 2 and the first session of Phase 3 in the SD and SΔ components in Experiment 1. Error bars represent standard error of the mean
The top panel of Fig. 3 displays mean target response rates in the SD and SΔ components across all sessions of Phase 3. A 2 × 5 (Component × Session) repeated-measures ANOVA conducted on these data revealed nonsignificant effects of Component, F(1, 4) = 2.58, p = .18, ηp2 = .39, and Session, F(4, 16) = 2.83, p = .06, ηp2 = .41, and a significant Component × Session interaction, F(4, 16) = 3.63, p = .03, ηp2 = .48. Simple effects tests conducted following the significant interaction revealed that target responding was higher in the SΔ compared with SD component in session 2 (p = .03) but the difference between components did not reach significance in the remaining sessions (p ≥ .09). Thus, there is not strong evidence to support differential target-response persistence across sessions of Phase 3.
Mean target (top panel) and alternative (bottom panel) responses per min across sessions of Phase 3 in the SD and SΔ components in Experiment 1. Error bars represent standard error of the mean
The bottom panel of Fig. 3 displays mean alternative response rates across sessions of Phase 3. A 2 × 5 (Component × Session) repeated-measures ANOVA conducted on these data revealed significant effects of Component, F(1, 4) = 49.64, p = .002, ηp2 = .93, and Session, F(4, 16) = 32.23, p < .001, ηp2 = .89, and a significant Component × Session interaction, F(4, 16) = 15.06, p < .001, ηp2 = .79. Simple effects tests conducted following the significant interaction revealed that alternative responding was higher in the SD compared with the SΔ component in the first (p < .001) and final (p = .04) sessions of Phase 3 and the mean difference between components was greater at the beginning of the phase (MD = 16.12, 95% CI [12.60, 19.64]) compared with the end (MD = 2.00, 95% CI [0.13, 3.87]). Thus, alternative responding was initially more elevated and subsequently decreased across sessions of Phase 3 in the SD component compared with the SΔ component.
To evaluate whether the alternative-response DI was significantly greater than 0.50 in Phase 3, DIs were first averaged across sessions of Phase 3 (M = 0.71, SEM = 0.04) to avoid conducting multiple analyses across sessions. Then, a one-sample t test was conducted on these data against the test value of 0.50. Alternative responding continued to be proportionally greater in the SD component in Phase 3, t(4) = 4.98, p = .008, d = 2.23, (see Table 1 for alternative response rates and corresponding DIs in the first session of Phase 3). Thus, even in the absence of reinforcer deliveries in the SD component during the resurgence test, rats continued to discriminate between the SD and SΔ components.
Discussion
The purpose of Experiment 1 was to determine whether an alternative-response SΔ would mitigate resurgence if the alternative response is also placed on extinction in the presence of the SD that had previously signaled alternative reinforcement availability. Following baseline in which target responding was reinforced, the target response was placed on extinction and discrimination of the alternative response was trained in a two-component multiple schedule in which alternative responding was reinforced in the SD component and extinguished in the SΔ component. Resurgence of target responding was then assessed in both components by placing the alternative response on extinction in the SD component.
Resurgence was observed in both components and was not reduced in the SΔ component compared with in the SD component. While it is not surprising that resurgence occurred following alternative-response extinction in the SD component, it is unclear why resurgence occurred in the SΔ component in which alternative reinforcement was never available. It seems unlikely that resurgence occurred in the SΔ component as a result of failure to effectively discriminate the stimuli arranged in Phase 2 given that alternative responding was differentially allocated across components during discrimination training, as measured by the DI. While it is possible that rats allocated behavior according to the signaling effects of the presence and absence of alternative reinforcement, the fact that the DI remained above indifference when alternative reinforcers were removed in Phase 3 suggests that the discriminative stimuli were contributing to response allocation to some extent.
Instead, resurgence may have occurred in the SΔ component as a result of the multiple schedule used during testing. That is, Fuhrman et al. (2016) and Fisher et al. (2020) evaluated resurgence in the presence or absence of only the SΔ stimulus, and while the purpose of Experiment 1 was to compare resurgence in both SD and SΔ components, it is possible that this difference contributed to the discrepant findings. It is well established that manipulating the reinforcement rate in one component of a multiple schedule can impact the response rate in the other component (e.g., Williams, 1983). Specifically, positive contrast refers to when behavior increases in an unchanged component following a decrease in the rate of reinforcement in the altered component (Reynolds, 1961a, 1961b). Thus, removing alternative reinforcement in one component of the multiple schedule in Phase 3 may have resulted in positive contrast.
In further support of this possibility, there is evidence to suggest that contrast effects may occur across concurrently available response options within a multiple schedule. For example, Catania (1961) evaluated contrast effects in concurrent multiple schedules using pigeons. Following a baseline in which pigeons earned food for pecking concurrently available red and green keys, a multiple schedule was introduced in which pecking the green key was placed on extinction in the first component and reinforced in the second component, while pecking the red key was reinforced in both components. Not surprisingly, green-key pecking in the unchanged component increased when extinction of green-key pecking was introduced in the first component. Interestingly, pecking the red key in the unchanged component also increased despite no changes in the contingencies for that response. These findings suggest that contrast effects may not be isolated to only the response in which the contingency was altered but may have a more general impact on behavior allocation within multiple schedules.
Based on these data, it may be the case that the increase in target responding in the SΔ component following extinction of the alternative response in the SD component in Phase 3 was the result of positive contrast. That is, target responding increased in the unchanged SΔ component following a decrease in the rate of reinforcement for the alternative response (i.e., VI 5 s to extinction) in the altered SD component. In fact, there is evidence that resurgence may be related to behavioral contrast. For example, Pyszczynski and Shahan (2013) observed resurgence of alcohol seeking in one component of a multiple schedule following extinction of food-maintained responding in the second component using rats. Following a baseline in which lever pressing produced alcohol in one component and chain pulling produced food in the second component, lever pressing was placed on extinction in the alcohol component in Phase 2. In the final phase, chain pulling was also placed on extinction in the food component and lever pressing in the alcohol component subsequently increased. The authors suggested that the resurgence effect observed in the alcohol component may have been the result of positive contrast.
Whether or not behavioral contrast contributed to our results, these data suggest that an SΔ stimulus may not mitigate resurgence if alternative-response extinction also occurs in the presence of the SD stimulus under multiple-schedule conditions. Taken together, the findings of Experiment 1 and those of the previous applied research in which resurgence was not observed when the SΔ was presented alone pose the question of what the necessary and sufficient conditions under which an SΔ will mitigate resurgence are.
Experiment 2
The purpose of Experiment 2 was to evaluate resurgence in the presence of the SD and SΔ in isolation and in the absence of discriminative stimuli altogether and to directly compare the effects of these conditions with the multiple-schedule test condition of Experiment 1. As in Experiment 1, target responding was reinforced in the first phase, and alternative response discrimination training and target-response extinction occurred in the second phase. Resurgence of target responding was assessed in the third phase across four groups of rats: three single-stimulus test groups and one multiple-stimulus test group. For the three single-stimulus tests, resurgence testing occurred in the presence of only the SΔ stimulus (i.e., SΔ Alone group), in the presence of only the SD stimulus (i.e., SD Alone group), or in the absence of both SΔ and SD stimuli (i.e., the No Stim group). For the Mult Stim group, resurgence testing occurred as in Experiment 1, in which resurgence was evaluated in both SΔ and SD components by placing the alternative response on extinction in the SD component.
Method
Subjects
Twenty-eight experimentally naïve male Long-Evans rats served as subjects. Rats were housed and cared for under the same conditions as in Experiment 1.
Apparatus
Five identical Med Associates operant chambers in addition to the five chambers from Experiment 1 were used.
Procedure
Experimental sessions were conducted in the same manner as in Experiment 1.
Training, Phase 1: Baseline, and Phase 2: Target extinction + alternative discrimination training
The procedures used in the training, and Phases 1 and 2 were identical to those described in Experiment 1 for rats in all groups. In brief, target lever pressing was reinforced on a VI 30-s schedule in Phase 1 for 20 sessions and then placed on extinction in Phase 2. During discrimination training, a two-component multiple schedule was introduced in which alternative lever pressing was reinforced on a VI 5-s schedule in the SD component and extinguished in the SΔ component. Components were differentially signaled by either a constant house light and tone or flashing house light and pulsing tone (on/off every 0.5 s), counterbalanced across rats. Phase 2 lasted 25 sessions.
Phase 3: Test
Prior to the start of this phase, rats were divided into four equal groups (n = 7) such that target response rates during the last three sessions of Phase 1 and the last three sessions of Phase 2 (within component) were not statistically different between groups. For all groups, sessions during this phase began as in Phase 2 (i.e., insertion of both the target and alternative levers and illumination of both lever lights). The target response remained on extinction and the alternative response was also placed on extinction for all groups, but the particular discriminative stimulus conditions present varied by group. The SΔ Alone, SD Alone, and No Stim groups were tested under single-stimulus conditions, and the Mult Stim group was tested under the same multiple-stimulus conditions as in Phase 2. Specifically, the SΔ stimulus established in discrimination training was presented continuously for the duration of the session for the SΔ Alone group, the SD stimulus was presented continuously for the SD Alone group, and all alternative-response discriminative stimuli were absent for the No Stim group. For example, the flashing house light and pulsing tone stimuli may have served as the SΔ stimulus and the constant house light/tone stimuli may have served as the SD stimulus for a particular rat. If this rat was assigned to the SΔ Alone group, the house light and tone would flash/pulse for the duration of the session, but if this rat was assigned to the SD Alone group, the house light and tone would remain on for the duration of the session. The house light and tone remained off for the duration of the session in the No Stim Test group, regardless of previous discriminative stimulus assignment. This phase lasted five sessions.
Data analyses
The primary dependent variables were target and alternative responses per min across sessions and between groups and components. Additionally, a discrimination index (DI) was calculated as in Experiment 1 to evaluate differential alternative-response allocation between discriminative stimuli during Phase 2 for all groups and during Phase 3 for the Mult Stim group. Greenhouse–Geisser corrections to degrees of freedom were applied when Mauchly’s test indicated a violation of sphericity for the within-subject factors in the analyses of variance (ANOVA). For all analyses, the within-subject factors included session and component, and the between-subject factor was group/test condition. Statistical significance was determined using α = .05 and a Bonferroni adjustment was applied to all simple effects tests.
Results
Table 2 provides a summary of mean response rates, discrimination indices, and reinforcer rates across phases for each group.
Phase 1: Baseline
Target responses per min increased across sessions of baseline to comparable levels for all groups. A one-way ANOVA conducted on the average target response rate from the last three sessions of baseline confirmed that there was no difference between groups, F(3, 24) = 0.04, p = .99, ηp2 < .01 (see Table 2).
Phase 2: Target extinction + alternative discrimination training
The top panel of Fig. 4 displays alternative response rates in the SD and SΔ components across sessions of Phase 2 for all groups. A 25 × 2 × 4 (Session × Component × Group) mixed-model ANOVA conducted on these data revealed significant effects of Session, F(2.54, 61.06) = 41.53, p < .001, ηp2 = .63, and Component, F(1, 24) = 141.36, p < .001, ηp2 = .85, and a significant Session × Component interaction, F(2.64, 63.36) = 36.39, p < .001, ηp2 = .60. The effect of Group, F(3, 24) = 0.12, p = .95, ηp2 = .01, and the Session × Group, F(7.63, 61.06) = 0.74, p = .94, ηp2 = .09, Component × Group, F(3, 24) = 0.14, p = .93, ηp2 = .018, and Session × Component × Group, F(7.92, 63.36) = 0.50, p = .99, ηp2 = .06, interactions were not significant. Simple effects tests conducted following the significant interaction revealed that alternative responding was significantly higher in the SD component in both the first (p < .001) and final sessions of Phase 2 (p < .001), but the mean difference between components was greater at the end of the phase (MD = 55.20, 95% CI [44.69, 66.35]) than the beginning (MD = 4.85, 95% CI [2.88, 6.82]). These results suggest that alternative responding increased across sessions to a higher level in the SD component and remained relatively low in the SΔ component, and this pattern did not vary by group (see Table 2 for Phase-2 terminal response rates). The lack of differences across groups was expected given that all groups were exposed to the same conditions in this phase.
Mean alternative (top panel) and target (bottom panel) responses per min in the SD (closed symbols and solid lines) and SΔ (open symbols and dashed lines) components across sessions of Phase 2 for each group in Experiment 2. All groups depicted experienced the same multiple schedule conditions during Phase 2 presented here and the group names refer to conditions that only differed in the following Phase-3 test presented in subsequent figures.Error bars represent standard error of the mean
Table 2 displays the alternative-response discrimination index (DI) for each group in the last session of Phase 2. A DI greater than 0.50 indicates that responding was proportionally higher in the SD component than in the SΔ component. To evaluate whether the DI for the last session of Phase 2 was significantly greater than 0.50, a one-way ANOVA was first conducted on these data to ensure no group differences, F(3, 24) = 0.13, p = .94, ηp2 = .02. Then, DIs were collapsed across groups to avoid conducting multiple analyses and a one-sample t-test was conducted on these data against the test value of 0.50. Alternative responding was proportionally higher in the SD component than in the SΔ component at the end of this phase, t(27) = 15.18, p < .001, d = 2.87. Taken together, the data in the top panel of Fig. 4 and the alternative-response DIs suggest that rats effectively allocated alternative responding according to the arranged discriminative stimuli.
The bottom panel of Fig. 4 displays target response rates in the SD and SΔ components across sessions of Phase 2 for each group. A 25 × 2 × 4 (Session × Component × Group) mixed-model ANOVA conducted on these data revealed significant effects of Session, F(3.28, 78.74) = 36.47, p < .001, ηp2 = .60, Component, F(1, 24) = 27.80, p < .001, ηp2 = .54, and a significant Session × Component interaction, F(5.08, 122.03) = 3.18, p = .009, ηp2 = .12. The effect of Group, F(3, 24) = 0.35, p = .79, ηp2 = .04, and the Session × Group, F(9.84, 78.74) = 0.63, p = .78, ηp2 = .07, Component × Group, F(3, 24) = 0.87, p = .47, ηp2 = .10, and Session × Component × Group, F(15.25, 122.03) = 1.03, p = .43, ηp2 = .11, interactions were not significant. Simple effects tests conducted following the significant Session × Component interaction revealed that target responding did not differ between components in the first session of Phase 2 (p = .64) but was significantly higher in the SΔ compared with the SD component in the final session (p < .001). These results suggest that target responding decreased relatively more in the SD component (in which reinforcement was available for the alternative response) and was elevated in the SΔ component (in which reinforcement for the alternative response was not available) at the end of this phase.
Phase 3: Test
Figure 5 displays target response rates in the last session of Phase 2 and the first session of Phase 3 across stimuli and groups. The left panel displays response rates in the SD and SΔ components during the last session of Phase 2 and the first session of Phase 3 for the Mult Stim group. The right panel displays response rates in the SD and SΔ components during the last session of Phase 2 and during the single stimulus condition in the first session of Phase 3 for the SD Alone, SΔ Alone, and No Stim groups.
Left panel: Mean target responses per min in the last session of Phase 2 and the first session of Phase 3 in the SD and SΔ components for the Mult Stim group. Right panel: Mean target responses per min in the last session of Phase 2 in the SD (black symbols) and SΔ components (white symbols) and the first session of Phase 3 under single stimulus testing (gray symbols) for the SD Alone, SΔ Alone, and No Stim groups. Solid line represents data paths between responding in SD component and Phase 3. Dotted lines represent data paths between responding in SΔ component and Phase 3. Error bars represent standard error of the mean
The resurgence analyses reported below were conducted in a series of steps informed by the purpose of Experiment 2. First, to evaluate resurgence under conditions comparable to those of Experiment 1, resurgence was compared between components for the Mult Stim group alone. Second, to evaluate the effect of the testing condition (i.e., multiple- or single-stimulus presentation), resurgence was compared between the Mult Stim and single-stimulus groups. Finally, to determine the effect of presenting the SD and SΔ stimulus in isolation and removal of the discriminative stimuli altogether, target responding was compared between the SD Alone, SΔ Alone, and No Stim groups. It is important to note that these Phase-3 data could not be analyzed in a single ANOVA because the groups differ in the number of factors. That is, the Mult Stim group has two within-subject factors (i.e., Component and Session) and no between-subject factors, whereas the single-stimulus test groups have one within-subject factor (i.e., Session) and one between-subject factor (i.e., Group).
First, to evaluate resurgence in the Mult Stim group alone, a 2 × 2 (Session × Component) repeated-measures ANOVA was conducted on target response rates in the SD and SΔ components across the last session of Phase 2 and the first session of Phase 3 for this group specifically. The effects of Session, F(1, 6) = 14.43, p = .008, ηp2 = .71, and Component, F(1, 6) = 12.34, p = .01, ηp2 = .67, and the Session × Component interaction, F(1, 6) = 6.87, p = .04, ηp2 = .53, were significant. Simple effects tests conducted following the significant Session × Component interaction revealed that target responding was significantly higher in the first session of Phase 3 relative to the last session of Phase 2 in the SD component (p = .005) but not in the SΔ component (p = .60). Thus, resurgence was only evident in the SD component for the Mult Stim group.
Second, to evaluate the impact of the testing condition (i.e., multiple- or single-stimulus presentation) on resurgence, target responding in the last session of Phase 2 and the first session of Phase 3 was compared between groups separately for SD and SΔ stimulus conditions. A 2 × 2 (Session × Group) mixed-model ANOVA was conducted on target response rates in the SD component of the last session of Phase 2 for both groups and in first session of Phase 3 for the SD Alone group and in the SD component of the first Phase-3 session for the Mult Stim group. The effect of Session, F(1, 12) = 22.25, p < .001, ηp2 = .65, was significant, and the effect of Group, F(1, 12) = 0.05, p = .82, ηp2 < .01, and the Session × Group interaction, F(1, 12) = 0.99, p = .34, ηp2 = .08, were not. A simple effects test following the significant effect of Session revealed that target responding was higher in the first session of Phase 3 relative to the last session of Phase 2 (p = .001), regardless of group. Thus, resurgence occurred under SD conditions and did not differ between multiple- and single-stimulus testing.
For analysis of SΔ conditions, a 2 × 2 (Session × Group) mixed-model ANOVA was conducted on target response rates in the SΔ component of the last session of Phase 2 for both groups and in first session of Phase 3 for the SΔ Alone group and in the SΔ component of the first Phase-3 session for the Mult Stim group. The effects of Session and Group, and the Session × Group interaction were not significant (p ≥ .13). These nonsignificant effects suggest that resurgence did not occur under either SΔ test.
Finally, target response rates in the first session of Phase 3 were compared between the SD Alone, SΔ Alone, and No Stim groups to evaluate the level of target responding in the presence and absence of alternative-response discriminative stimuli. A one-way ANOVA conducted on these data revealed a nonsignificant effect of Group, F(2, 18) = 0.34, p = .72, ηp2 = .04, suggesting that target response rates in the first session of resurgence testing were comparable between the three single-stimulus groups (see Table 2).
In summary of the above resurgence analyses, target responding did not differ by group at the end of Phase 2 but was overall higher in the SΔ component than in the SD component. Subsequently, resurgence occurred in the presence of the SD stimulus and not the SΔ stimulus, regardless of test condition. Additionally, presentation of the SD and SΔ stimulus alone, or removal of discriminative stimuli altogether did not differentially affect target responding in the first test session of Phase 3.
Figure 6 displays target and alternative response rates across all sessions of Phase 3, separated by multiple- and single-test conditions. The left panels show target and alternative responding in the SD and SΔ components for the Mult Stim group. The right panels show target and alternative responding for the SD Alone, SΔ Alone, and No Stim groups. Analyses of target and alternative responding across sessions of Phase 3 were conducted in a series of steps similar to the resurgence analyses reported above. First, responding was compared between components for the Mult Stim group alone. Second, responding was compared between the three Single Stim groups. Finally, to evaluate the effect of testing condition, responding was compared between multiple- and single-stimulus conditions.
Mean target (top panels) and alternative (bottom panels) responses per min across sessions of Phase 3 in the SD and SΔ components for the Mult Stim group (left panels) and for the SD Alone, SΔ Alone, and No Stim single-stimulus test groups (right panels). Error bars represent standard error of the mean
To evaluate target responding in the Mult Stim group alone, a 2 × 5 (Component × Session) repeated-measures ANOVA was conducted on target responding across sessions of Phase 3 for this group. The effect of Session, F(1.53, 9.18) = 8.40, p = .01, ηp2 = .58, was significant, while the effect of Component, F(1, 6) = 2.57, p = .16, ηp2 = .30, and the Component × Session interaction, F(4, 24) = 1.29, p = .30, ηp2 = .18, were not. A simple effects test following the significant effect of Session revealed that target responding was higher in the first session relative to the last session of Phase 3 (p = .02), regardless of component. Thus, target responding significantly decreased across sessions in Phase 3 for the Mult Stim group, and this change did not differ between components.
To evaluate target responding under single-stimulus testing conditions, a 3 × 5 (Group × Session) mixed-model ANOVA was conducted on target responding across sessions of Phase 3 for the SD Alone, SΔ Alone, and No Stim groups. The effect of Session, F(1.65, 29.67) = 11.44, p < .001, ηp2 = .39, was significant, and the effect of Group, F(2, 18) = 0.26, p = .78, ηp2 = .03, and Group × Session interaction, F(3.30, 29.67) = 0.88, p = .47, ηp2 = .09, were not. A simple effects test following the significant effect of Session revealed that target responding was higher in the first session relative to the last session of Phase 3 (p = .01), regardless of group. Thus, target responding significantly decreased across sessions in Phase 3 under single-stimulus test conditions, and this change did not differ between the SD Alone, SΔ Alone, and No Stim groups.
To evaluate target responding across sessions of Phase 3 between the multiple- and single-stimulus testing conditions, target response rates were collapsed across components for the Mult Stim group and across groups for the SD Alone, SΔ Alone, and No Stim groups. These data were collapsed in this manner given the nonsignificant effects of Component for the Mult Stim group and the nonsignificant effects of Group for the single-stimulus groups reported above. A 2 × 5 (Test Condition × Session) mixed-model ANOVA conducted on these data revealed a significant effect of Session, F(1.72, 44.70) = 13.97, p < .001, ηp2 = .35, and a nonsignificant effect of Test Condition, F(1, 26) = 0.21, p = .65, ηp2 < .01, and Test Condition × Session interaction, F(1.72, 44.70) = 0.22, p = .77, ηp2 < .01. A simple effects test following the significant effect of Session revealed that target responding was higher in the first session relative to the last session of Phase 3 (p = .003), regardless of test condition. Thus, target responding significantly decreased across sessions in Phase 3 and did not differ between multiple- and single-stimulus testing conditions.
To evaluate alternative responding in the Mult Stim group alone, a 2 × 5 (Component × Session) repeated-measures ANOVA was conducted on alternative responding across sessions of Phase 3 for this group. The effects of Component, F(1, 6) = 30.14, p = .002, ηp2 = .83, and Session, F(4, 24) = 40.87, p < .001, ηp2 = .87, and the Component × Session interaction, F(4, 24) = 11.40, p < .001, ηp2 = .66, were all significant. Simple effects tests conducted following the significant interaction revealed that alternative responding was significantly higher in the SD compared with the SΔ component in both the first (p = .004) and last sessions of Phase 3 (p = .02), and the mean difference between components was greater at the beginning of the phase (MD = 17.02, 95% CI [7.89, 26.16]) compared with the end (MD = 1.51, 95% CI [0.41, 2.61]). Thus, alternative responding was initially more elevated and subsequently decreased more steeply across sessions of Phase 3 in the SD component than in the SΔ component for the Mult Stim group. Additionally, the DI averaged across these sessions (M = 0.70, SEM = 0.04) was statistically greater than 0.50, t(6) = 5.28, p < .001, d = 1.99, suggesting that differential alternative responding between components continued during Phase 3 for the Mult Stim group.
To evaluate alternative responding under single-stimulus testing conditions, a 3 × 5 (Group × Session) mixed-model ANOVA was conducted on alternative responding across sessions of Phase 3 for the SD Alone, SΔ Alone, and No Stim groups. The effect of Session, F(1.61, 28.93) = 54.19, p < .001, ηp2 = .75, was significant, and the effect of Group, F(2, 18) = 0.06, p = .94, ηp2 < .01, and the Group × Session interaction, F(3.21, 28.93) = 1.27, p = .31, ηp2 = .12, were not. A simple effects test following the significant effect of Session revealed that alternative responding was higher in the first session relative to the last session of Phase 3 (p < .001), regardless of group. Thus, alternative responding significantly decreased across sessions in Phase 3 under single-stimulus test conditions, and this change did not differ between the SD Alone, SΔ Alone, and No Stim groups.
To evaluate the impact of multiple-stimulus and single-stimulus testing conditions on alternative responding during extinction, alternative response rates across sessions of Phase 3 were compared between groups under comparable stimulus conditions. A 2 × 5 (Group × Session) mixed-model ANOVA was conducted on alternative responding across sessions of Phase 3 in the SD component of the Mult Stim group and across sessions in the SD Alone group. The effect of Session, F(4, 48) = 63.30, p < .001, ηp2 = .84, and the Group × Session interaction, F(4, 48) = 3.87, p = .008, ηp2 = .24, were significant, and the effect of Group, F(1, 12) = 2.52, p = .14, ηp2 = .17, was not. Simple effects tests conducted following the significant interaction revealed that alternative responding was significantly higher in the Mult Stim group compared with the SD Alone group during the first session of Phase 3 (p = .04) and that responding did not differ between groups at the end of the phase (p = .60). These results suggest that alternative responding in the presence of the SD stimulus was initially more elevated and subsequently decreased across sessions of Phase 3 in the multiple-stimulus test compared with the single-stimulus test.
For analysis of SΔ conditions, a 2 × 5 (Group × Session) mixed-model ANOVA was conducted on alternative responding across sessions of Phase 3 in the SΔ component of the Mult Stim group and across sessions in the SΔ Alone group. The effect of Session, F(1.69, 20.23) = 37.46, p < .001, ηp2 = .76, and the Group × Session interaction, F(1.69, 20.23) = 4.84, p = .02, ηp2 = .29, were significant, and the effect of Group, F(1, 12) = 3.96, p = .07, ηp2 = .25, was not. Simple effects tests conducted following the significant interaction revealed that alternative responding was significantly lower for the Mult Stim group compared with the SΔ Alone group during the first session of Phase 3 (p = .03), and that responding did not differ between groups at the end of the phase (p = .22). These results suggest that alternative responding in the presence of the SΔ stimulus was less persistent in the multiple-stimulus test than in the single-stimulus test. Thus, differential alternative-response extinction in the presence of the SD and SΔ stimuli was only evident in the multiple-stimulus test condition and not under single-stimulus testing (see Table 2 for alternative response rates across stimulus conditions in the first session of Phase 3).
Discussion
The purpose of Experiment 2 was to compare resurgence under the multiple-stimulus testing condition of Experiment 1 in which the SD and SΔ were presented in a multiple schedule to single-stimulus testing conditions in which the SD and SΔ were presented in isolation or removed altogether. Baseline and alterative-response discrimination training occurred as in Experiment 1, and resurgence of target responding was assessed in a multiple-schedule for one group of rats and, for the remaining three groups, resurgence was tested in presence of either the SΔ stimulus alone, the SD stimulus alone, or no discriminative stimuli.
The results of Experiment 2 suggest that target behavior was not differentially impacted by testing condition. That is, regardless of testing under a multiple-schedule or in the presence of a single discriminative stimulus, resurgence was evident in the SD, but target responding did not significantly increase across phases in the SΔ. These resurgence results are inconsistent with the findings from Experiment 1, in which target responding did significantly increase across phases in both the SD and SΔ. Given that the discrimination training procedures were identical between experiments, as well as the testing procedures in Experiment 1 and for the Mult Stim group of Experiment 2, it is unclear what contributed to this discrepancy. It should be noted that the sample size in Experiment 2 (N = 28) was larger than that of Experiment 1 (N = 5), and as a result, it is possible that differences in sampling contributed to this inconsistent finding.
While resurgence was not evident under SΔ conditions in Experiment 2, it is important to note that target responding was significantly elevated in the SΔ component relative to the SD component during the last session of Phase 2 (see Figs. 4 and 5). Further, target response rates were not different between stimuli across sessions of resurgence testing (see Fig. 6). Thus, while target responding did not necessarily increase across phases, it is clear that the SΔ did not significantly reduce target responding during resurgence testing.
This pattern of target responding resembles those reported in which parameters of alternative reinforcement, such as rate and magnitude, are manipulated (Bouton & Trask, 2016; Craig et al., 2016; Craig, Browning, Nall, et al., 2017; Craig & Shahan, 2016; Sweeney & Shahan, 2013a). For example, Craig and Shahan (2016) reported elevated target response rates during Phase 2 in rats that received a relatively lean rate of alternative reinforcement compared with rats that received a relatively rich rate. Further, the groups that had received rich alternative reinforcement showed resurgence while the groups that had received lean reinforcement did not, but target responding did not differ between groups in Phase 3. Thus, parameters of alternative reinforcement as well as stimuli that differentially signal alternative reinforcement both contribute to levels of target responding across Phases 2 and 3. Additionally, the extent to which target responding is elevated during treatment is related to whether or not target responding necessarily increases (Shahan & Craig, 2017).
Additionally, target response rates in Phase 3 for the No Stim group were also comparable to those in the Single and Mult SD and SΔ conditions, suggesting that overall levels of target responding during testing was not differentially affected by the presence or absence of alternative-response discriminative stimuli. From an applied perspective, this may suggest that a treatment adherence failure in which the FCR discriminative stimuli are completely absent may not necessarily result in greater resurgence when the FCR contacts extinction. This finding is somewhat surprising given that there is evidence to suggest that removing both alternative reinforcers and discriminative stimuli produces greater relapse. For example, Podlesnik and Kelley (2014) observed greater resurgence of key pecking in pigeons following removal of alternative reinforcement when the alternative-response discriminative stimulus (i.e., an illuminated key) was absent (i.e., key was darkened) compared with when it remained present during resurgence testing. More broadly, the results of Podlesnik and Kelley (2014) may be related to experiments in which resurgence and renewal procedures are combined. That is, renewal refers to the increase in behavior following a change in the context in which that behavior was previously extinguished (Bouton et al., 2011). In ABA renewal, a response is reinforced during baseline in a particular context (i.e., Context A), that response is placed on extinction in a separate context (i.e., Context B), and relapse is tested in the original baseline context. There is some evidence to suggest that superimposing such contextual manipulations onto a resurgence procedure results in greater relapse effects (Kincaid et al., 2015; Trask & Bouton, 2016).
In relation to Experiment 2, the Phase 3 test for the No Stim group might approximate a return to the baseline context (i.e., Context A) because the alternative-response discriminative stimuli were absent in both phases. Further, the presence of discriminative stimuli during discrimination training could be characterized as Context B. Based on the findings described above, resurgence might be expected to be largest in this group. While the average target response rate in the first session of Phase 3 was numerically highest in this group (see Table 2), this effect was not significant. Whether or not this is inconsistent with the resurgence + renewal literature is unclear given the mixed results reported in the literature (e.g., Nighbor et al., 2018; Sweeney & Shahan, 2015).
While testing condition did not have an effect on target response rates in Phase 3, persistence of alternative responding during extinction was differentially impacted by multiple- and single-stimulus test conditions. Specifically, alternative response rates across sessions of Phase 3 were more elevated in the SD component than in the SΔ component in the Mult Stim group, but alternative-response extinction was comparable between the SD Alone, SΔ Alone, and No Stim groups. Furthermore, alternative response rates were higher in the SD component and lower in the SΔ component for the Mult Stim group compared with the single-stimulus groups. Thus, the discriminative stimuli contributed to differential alternative-response allocation during extinction in the multiple schedule, but this differentiation was not evident between groups in the single-stimulus conditions.
These results may be related to the differential resistance to extinction observed in multiple schedules, but not in single schedules. Cohen (1998) reported more resistance to extinction in a stimulus context associated with a richer rate of reinforcement compared with a stimulus context associated with a leaner rate only if the stimuli were presented within a multiple schedule but not when presented in isolation in a single schedule. These findings suggest that the comparison of discriminative stimuli inherent in a multiple schedule may be important for differential response allocation under extinction. Thus, it is possible that comparison of SD and SΔ stimuli within the multiple schedule contributed to differential alternative-response persistence in Phase 3 in the Mult Stim group compared with the single stimulus presentation (or absence) in the other groups.
Given that one of the goals of discrimination training in the clinic is to control the overall rates of the FCR and prevent resurgence of challenging behavior (Saini et al., 2016), it would be ideal that the FCR persists during extended periods of extinction under SD but not SΔ conditions. Fisher et al. (2020) observed lower rates of the FCR during the extinction challenge when the SΔ stimulus was present compared with when it was absent for three participants and found no difference for the fourth participant, and Fuhrman et al. (2016) observed differential rates of the FCR between conditions in one participant, but not the other. Thus, there is generally more evidence that following FCT, the FCR is less persistent when the SΔ stimulus is presented alone compared with when it is absent, but SD tests were not included. Additionally, the nondifferential alternative-response extinction obtained in the single-stimulus tests of the current experiment is not entirely consistent with these findings. As a result, it is unclear whether to expect greater FCR persistence in the face of extinction under SD conditions.
In summary, the results of Experiment 2 suggest that target behavior was not significantly reduced in the presence of a stimulus that signaled alternative-response extinction regardless if that stimulus was presented in isolation or alternated with a stimulus that signaled alternative reinforcement. This conclusion is overall consistent with the results from Experiment 1, but are inconsistent with those reported in the applied literature. Thus, this discrepancy is not likely due to the difference in the testing condition between studies. Instead, differences in the discrimination training procedures may be responsible.
General discussion
Previous applied research has reported significant reductions in resurgence of severe destructive behavior in the presence of a stimulus that signals alternative-response extinction (i.e., an SΔ). The purpose of Experiment 1 was to test the generality of this finding by determining whether an alternative-response SΔ would mitigate resurgence of target responding when the alternative response also contacts extinction under SD conditions that had previously signaled the availability of reinforcement for the alternative response. Resurgence of target responding was comparable in the SD and SΔ stimulus conditions. These results conflict with those previously reported and suggest that the conditions under which an SΔ stimulus may prevent or mitigate resurgence are limited; however, given the testing conditions used in the applied research, it is possible that an SΔ stimulus may only prevent resurgence when presented in insolation, and not when presented in close temporal proximity to the SD stimulus within a multiple schedule.
The purpose of Experiment 2 was to determine the independent effects of alternative-response discriminative stimuli on resurgence of target responding, and to compare these effects to those produced by discriminative-stimuli presented within a multiple schedule. Resurgence was evident in the SD, but target responding remained elevated and did not increase across phases in the SΔ, and this effect was consistent in both the single-stimulus and multiple-stimulus test conditions. Additionally, rates of target responding during resurgence testing were not differentially affected by the SD stimulus, SΔ stimulus, or the absence of discriminative stimuli altogether.
The overall pattern of target and alternative responding during discrimination training was consistent between Experiments 1 and 2. That is, alternative responding was allocated according to the arranged discrimination as measured by the discrimination index (DI), but target responding remained generally elevated in the SΔ component compared with the SD. Additionally, resurgence occurred under SD conditions in both experiments, but resurgence occurred under SΔ conditions only in Experiment 1. It is possible that the effect observed in Experiment 1 was due to positive contrast; however, because this effect was not evident in the Mult Stim group of Experiment 2, the exact role of behavioral contrast remains unclear. As mentioned in the discussion of Experiment 2, this discrepancy between experiments may be due to differences in the sample size.
Importantly, the failure to observe an increase in target responding across phases in the SΔ in Experiment 2 was not likely the result of any mitigating effect of the SΔ, but rather the significantly elevated levels of target responding in the SΔ component at the end of Phase 2. In contrast, target response rates at the end of Phase 2 in both Fuhrman et al. (2016) and Fisher et al. (2020) were zero or near zero prior to resurgence testing. It is important to note that a changeover delay (COD) for switching from the SΔ to the SD component was programmed for both alternative and target responses in these studies. As a result, the SΔ may have come to signal both alternative-response and target-response extinction thereby contributing to the reduction of target responding and it is unclear whether the SΔ mitigated resurgence because it signaled extinction of the alternative response, target response, or both. To avoid this confound and to evaluate the effects of a discriminative stimulus that only signaled alternative-response extinction, a COD for switching from the SΔ to the SD component was programmed for only the alternative response, and not the target response in Experiments 1 and 2.
Overall, it is clear that the alternative-response SΔ stimulus did not significantly reduce target responding during resurgence testing in either experiment. That is, across experiments, target responding was either higher in the SΔ compared with the SD, or comparable between SΔ and SD stimuli across sessions of Phase 3. Importantly, it is not likely that this finding was due to a failure to effectively discriminate the SD and SΔ stimuli during resurgence testing. Specifically, alternative responding continued to be differentially allocated according to the discriminative stimuli (i.e., DI > 0.50) when alternative reinforcement was removed in Phase 3 in Experiment 1 and in the Mult Stim group of Experiment 2. Thus, the question remains: what are the necessary and sufficient conditions under which an SΔ will mitigate resurgence following discrimination training?
In addition to teaching the client to discriminate when FCR reinforcement is and is not available, discrimination training is an effective means to thin the rate of FCR reinforcement, thereby further increasing the feasibility of treatment implementation (e.g., Betz et al., 2013). Fuhrman et al. (2016) and Fisher et al. (2020) included FCR reinforcement rate thinning during discrimination training by increasing the duration of the SΔ component. Initially the duration of the components were 60 s and 30 s and were increased to 60 s and 240 s for the SD and SΔ components, respectively. As a result, participants in both studies experienced an SΔ component that was relatively longer than the SD component by the time resurgence was tested.
Further, there is evidence to suggest that the duration of exposure to the SΔ stimulus contributes to effective discrimination. For example, Andrzejewski et al. (2007) evaluated the impact of the length of exposure to the SΔ stimulus on the acquisition of a discriminated responding in rats. In a two-component multiple schedule, the duration of the SD component was held constant at 2 min and the duration of the SΔ component was either 1 or 4 min. Regardless of the rate of reinforcement in the SD component, the speed of acquisition of the discrimination (as evident by proportion of responding in SD) was substantially faster when the duration of the SΔ component was 4 min compared with 1 min. Additionally, Kalmbach et al. (2019) evaluated the effect of SΔ duration on response suppression in the presence of the SΔ relative to its absence in mice. The duration of the SΔ component was either 20, 40 or 80 s across groups, and the duration of the absence of the SΔ was held at an average of 40 s. Similarly, to the findings reported by Andrzejewski et al. (2007), response suppression was a direct function of the SΔ duration such that longer durations produced greater suppression and better discrimination.
In light of these data, it is possible that increasing the duration of the SΔ component during FCT as a means of thinning rate of FCR reinforcement contributed to the reduced resurgence observed by Fuhrman et al. (2016) and Fisher et al. (2020). Importantly, in the present experiments, the duration of the SΔ component was the same as the SD component and was not increased at any point during discrimination training. Further, there is some evidence for increased alternative-response discrimination in the applied studies compared with the present experiments. For example, rates of the FCR decreased for all but one participant across the two applied studies when the duration of the SΔ component increased during discrimination training, suggesting further response suppression, whereas alternative response rates in the SΔ component remained constant across Phase 2 in Experiments 1 and 2. Additionally, rates of the FCR were lower during extinction in the presence of the SΔ stimulus compared with in its absence in the applied studies while alternative responding during extinction in the present experiments was not differentially affected by the SD or SΔ stimuli alone or by the absence of discriminative stimuli altogether. However, these comparisons only provide tentative evidence to suggest the increased SΔ duration is a critical variable. Currently, there is no empirical evidence for a casual relation between SΔ duration, alternative-response discrimination, and subsequent resurgence. Future research may be directed toward systematically evaluating the effects of increasing the duration of alternative-response SΔ stimulus presentation on resurgence.
Along with the duration of the SΔ, other procedural differences between the present and applied experiments may have also contributed to the discrepant findings. In both Fuhrman et al. (2016) and Fisher et al. (2020), participants were given verbal descriptions of the contingencies signaled by the SD and SΔ stimuli prior to each session during discrimination training, and more extensive training was administered for individual participants if necessary. Fisher et al. also conducted pretraining procedures to program errorless acquisition of the FCR and to establish discriminative control of the SD and SΔ prior to the resurgence procedure and no such pretraining was implemented in the present experiments. Lastly, the length of Phase 2 in Experiments 1 and 2 was longer than the length of FCT across participants in both applied studies. While increased in treatment duration produces small decreases in resurgence in rats (Shahan et al., 2020), this effect is not evident in the clinic (Greer et al., 2020). Overall, the extent to which these differences contributed to the obtained findings is unknown and warrants future investigation.
Translational research considers the applicability of fundamental behavioral principles to issues of social significance. Specifically, bidirectional translational research uses clinically significant questions to inform basic research which in turn improves future clinical research and practice (Mace & Critchfield, 2010). The present experiments provide additional support for the utility of translational research, and the obtained findings suggest that the conditions under which an alternative-response SΔ stimulus will successfully prevent resurgence are limited. While future research is certainly warranted, the present experiments are an initial step toward a more comprehensive understanding of the relation between alternative-response discrimination training and resurgence.
Data availability
Data are available from the first author upon request. Experiments have not been preregistered.
References
Andrzejewski, M. E., Ryals, C. D., Higgins, S., Sulkowski, J., Doney, J., Kelley, A. E., & Bersh, P. J. (2007). Is extinction the hallmark of operant discrimination? Reinforcement and SΔ effects. Behavioural Processes, 74(1), 49–63. https://doi.org/10.1016/j.beproc.2006.09.010
Balsam, P. D. (1988). Selection, representation, and equivalence of controlling stimuli. In R. C. Atkinson, R. J. Herrnstein, G. Lindzey, & R. D. Luce (Eds.), Stevens’ handbook of experimental psychology: Perception and motivation; Learning and cognition (pp. 111–166). Wiley.
Bernal-Gamboa, R., Gámez, A. M., & Nieto, J. (2017). Reducing spontaneous recovery and reinstatement of operant performance through extinction-cues. Behavioural Processes, 135, 1–7. https://doi.org/10.1016/j.beproc.2016.11.010
Betz, A. M., Fisher, W. W., Roane, H. S., Mintz, J. C., & Owen, T. M. (2013). A component analysis of schedule thinning during functional communication training. Journal of Applied Behavior Analysis, 46(1), 219–241. https://doi.org/10.1002/jaba.23
Bouton, M. E., Todd, T. P., Vurbic, D., & Winterbauer, N. E. (2011). Renewal after the extinction of free operant behavior. Learning & Behavior, 39(1), 57–67. https://doi.org/10.3758/s13420-011-0018-6
Bouton, M. E., & Trask, S. (2016). Role of the discriminative properties of the reinforcer in resurgence. Learning & Behavior, 44(2), 137–150. https://doi.org/10.3758/s13420-015-0197-7
Briggs, A. M., Fisher, W. W., Greer, B. D., & Kimball, R. T. (2018). Prevalence of resurgence of destructive behavior when thinning reinforcement schedules during functional communication training. Journal of Applied Behavior Analysis, 51(3), 620–633. https://doi.org/10.1002/jaba.472
Carr, E. G., & Durand, V. M. (1985). Reducing behavior problems through functional communication training. Journal of Applied Behavior Analysis, 18(2), 111–126. https://doi.org/10.1901/jaba.1985.18-111
Catania, A. C. (1961). Behavioral contrast in a multiple and concurrent schedule of reinforcement. Journal of the Experimental Analysis of Behavior, 4(4), 335–342. https://doi.org/10.1901/jeab.1961.4-335
Cohen, S. L. (1998). Behavioral momentum: The effects of the temporal separation of rates of reinforcement. Journal of the Experimental Analysis of Behavior, 69(1), 29–47. https://doi.org/10.1901/jeab.1998.69-29
Craig, A. R., Browning, K. O., Nall, R. W., Marshall, C. M., & Shahan, T. A. (2017). Resurgence and alternative-reinforcer magnitude. Journal of the Experimental Analysis of Behavior, 107(2), 218-233. https://doi.org/10.1002/jeab.245
Craig, A. R., Browning, K. O., & Shahan, T. A. (2017). Stimuli previously associated with reinforcement mitigate resurgence. Journal of the Experimental Analysis of Behavior, 108(2), 139-150. https://doi.org/10.1002/jeab.278
Craig, A. R., Nall, R. W., Madden, G. J., & Shahan, T. A. (2016). Higher rate alternative non-drug reinforcement produces faster suppression of cocaine seeking but more resurgence when removed. Behavioural Brain Research, 306(1), 48-51. https://doi.org/10.1016/j.bbr.2016.03.026
Craig, A. R., & Shahan, T. A. (2016). Behavioral momentum theory fails to account for the effects of reinforcement rate on resurgence. Journal of the Experimental Analysis of Behavior, 105(3), 375–392. https://doi.org/10.1002/jeab.207
Durand, V. M., & Moskowitz, L. (2015). Functional communication training: Thirty years of treating challenging behavior. Topics in Early Childhood Special Education, 35(2), 116–126. https://doi.org/10.1177/0271121415569509
Emerson, E., Kiernan, C., Alborz, A., Reeves, D., Mason, H., Swarbrick, R., Mason, L., & Hatton, C. (2001). The prevalence of challenging behaviors: A total population study. Research in Developmental Disabilities, 22(1), 77–93. https://doi.org/10.1016/S0891-4222(00)00061-5
Epstein, R. (1985). Extinction-induced resurgence: Preliminary investigations and possible applications. The Psychological Record, 35(2), 143–153. https://doi.org/10.1007/BF03394918
Fisher, W. W., Fuhrman, A. M., Greer, B. D., Mitteer, D. R., & Piazza, C. C. (2020). Mitigating resurgence of destructive behavior using the discriminative stimuli of a multiple schedule. Journal of the Experimental Analysis of Behavior. 113(1), 263-277. https://doi.org/10.1002/jeab.552
Fleshler, M., & Hoffman, H. S. (1962). A progression for generating variable‐interval schedules. Journal of the Experimental Analysis of Behavior, 5(4), 529–530. https://doi.org/10.1901/jeab.1962.5-529
Fuhrman, A. M., Fisher, W. W., & Greer, B. D. (2016). A preliminary investigation on improving functional communication training by mitigating resurgence of destructive behavior. Journal of Applied Behavior Analysis, 49(4), 884–899. https://doi.org/10.1002/jaba.338
Greer, B. D., Fisher, W. W., Retzlaff, B. J., & Fuhrman, A. M. (2020). A preliminary evaluation of treatment duration on the resurgence of destructive behavior. Journal of the Experimental Analysis of Behavior, 113(1), 251–262. https://doi.org/10.1002/jeab.567
Greer, B. D., Mitteer, D. R., & Fisher, W. W. (2018). A practical approach to functional communication training. In A. C. Sella & D. M. Ribeiro (Eds.), Behavior analysis applied to autism spectrum disorders. Appris.
Greer, B. D., & Shahan, T. A. (2019). Resurgence as choice: Implications for promoting durable behavior change. Journal of Applied Behavior Analysis, 52(3), 816–846. https://doi.org/10.1002/jaba.573
Hanley, G. P., Iwata, B. A., & Thompson, R. H. (2001). Reinforcement schedule thinning following treatment with functional communication training. Journal of Applied Behavior Analysis, 34(1), 17–38. https://doi.org/10.1901/jaba.2001.34-17
Harvey, S. T., Boer, D., Meyer, L. H., & Evans, I. M. (2009). Updating a meta-analysis of intervention research with challenging behaviour: Treatment validity and standards of practice. Journal of Intellectual and Developmental Disability, 34(1), 67–80. https://doi.org/10.1080/13668250802690922
Iwata, B. A., Dorsey, M. F., Slifer, K. J., Bauman, K. E., & Richman, G. S. (1994). Toward a functional analysis of self-injury. Journal of Applied Behavior Analysis, 27(2), 197–209. https://doi.org/10.1901/jaba.1994.27-197
Kalmbach, A., Chun, E., Taylor, K., Gallistel, C. R., & Balsam, P. D. (2019). Time-scale-invariant information-theoretic contingencies in discrimination learning. Journal of Experimental Psychology: Animal Learning and Cognition, 45(3), 280–289. https://doi.org/10.1037/xan0000205
Kincaid, S. L., Lattal, K. A., & Spence, J. (2015). Super-resurgence: ABA renewal increases resurgence. Behavioural Processes, 115, 70–73. https://doi.org/10.1016/j.beproc.2015.02.013
Kurtz, P. F., Boelter, E. W., Jarmolowicz, D. P., Chin, M. D., & Hagopian, L. P. (2011). An analysis of functional communication training as an empirically supported treatment for problem behavior displayed by individuals with intellectual disabilities. Research in Developmental Disabilities, 32(6), 2935–2942. https://doi.org/10.1016/j.ridd.2011.05.009
Lattal, K. A., & Wacker, D. (2015). Some dimensions of recurrent operant behavior. Mexican Journal of Behavior Analysis, 41(2), 1–13. https://doi.org/10.5514/rmac.v41.i2.63716
Mace, F. C., & Critchfield, T. S. (2010). Translational research in behavior analysis: Historical traditions and imperative for the future. Journal of the Experimental Analysis of Behavior, 93(3), 293-312. https://doi.org/10.1901/jeab.2010.93-293
Marsteller, T. M., & St Peter, C. C. (2012). Resurgence during treatment challenges. Mexican Journal of Behavior Analysis, 38(1), 7–23. https://www.redalyc.org/articulo.oa?id=593/59335804002
Nieto, J., Uengoer, M., & Bernal-Gamboa, R. (2017). A reminder of extinction reduces relapse in an animal model of voluntary behavior. Learning & Memory, 24(2), 76–80. https://doi.org/10.1101/lm.044495.116
Nighbor, T. D., Kincaid, S. L., O’Hearn, C. M., & Lattal, K. A. (2018). Stimulus contributions to operant resurgence. Journal of the Experimental Analysis of Behavior, 110(2), 243–251. https://doi.org/10.1002/jeab.463
Podlesnik, C. A., & Kelley, M. E. (2014). Resurgence: Response competition, stimulus control, and reinforcer control. Journal of the Experimental Analysis of Behavior, 102(2), 231–240. https://doi.org/10.1002/jeab.102
Pyszczynski, A. D., & Shahan, T. A. (2013). Loss of nondrug reinforcement in one context produces alcohol seeking in another context. Behavioural pharmacology, 24(5/6), 496–503. https://doi.org/10.1097/FBP.0b013e328364502a
Reynolds, G. S. (1961a). An analysis of interactions in a multiple schedule. Journal of the Experimental Analysis of Behavior, 4(2), 107–117. https://doi.org/10.1901/jeab.1961.4-107
Reynolds, G. S. (1961b). Behavioral contrast. Journal of the Experimental Analysis of Behavior, 4(1), 57–71. https://doi.org/10.1901/jeab.1961.4-57
Rilling, M. (1977). Stimulus control and inhibitory processes. In W. K. Honig & J. E. Staddon (Eds.), Handbook of operant behavior (pp. 432–480). Prentice-Hall.
Saini, V., Miller, S. A., & Fisher, W. W. (2016). Multiple schedules in practical application: Research trends and implications for future investigation. Journal of Applied Behavior Analysis, 49(2), 421–444. https://doi.org/10.1002/jaba.300
Schepers, S. T., & Bouton, M. E. (2015). Effects of reinforcer distribution during response elimination on resurgence of an instrumental behavior. Journal of Experimental Psychology: Animal Learning and Cognition, 41(2), 179–192. https://doi.org/10.1037/xan0000061
Shahan, T. A. (2002). Observing behavior: Effects of rate and magnitude of primary reinforcement. Journal of the Experimental Analysis of behavior, 78(2), 161–178. https://doi.org/10.1901/jeab.2002.78-161
Shahan, T. A., Browning, K. O., & Nall, R. W. (2020). Resurgence as choice in context: Treatment duration and on/off alternative reinforcement. Journal of the Experimental Analysis of Behavior. 113(1), 57-76. https://doi.org/10.1002/jeab.563
Shahan, T. A., & Craig, A. R. (2017). Resurgence as choice. Behavioural Processes, 141, 100–127. https://doi.org/10.1016/j.beproc.2016.10.006
St. Peter, C. C. (2015). Six reasons why applied behavior analysts should know about resurgence. Mexican Journal of Behavior, 41(2), 252–268. https://doi.org/10.5514/rmac.v41.i2.63775
St. Peter Pipkin, C., Vollmer, T. R., & Sloman, K. N. (2010). Effects of treatment integrity failures during differential reinforcement of alternative behavior: A translational model. Journal of Applied Behavior Analysis, 43(1), 47-70. https://doi.org/10.1901/jaba.2010.43-47
Sweeney, M. M., & Shahan, T. A. (2013a). Effects of high, low, and thinning rates of alternative reinforcement on response elimination and resurgence. Journal of the Experimental Analysis of Behavior, 100(1), 102–116. https://doi.org/10.1002/jeab.26
Sweeney, M. M., & Shahan, T. A. (2013b). Behavioral momentum and resurgence: Effects of time in extinction and repeated resurgence tests. Learning & Behavior, 41(4), 414–424. https://doi.org/10.3758/s13420-013-0116-8
Sweeney, M. M., & Shahan, T. A. (2015). Renewal, resurgence, and alternative reinforcement context. Behavioural Processes, 116, 43–49. https://doi.org/10.1016/j.beproc.2015.04.015
Thrailkill, E. A., Ameden, W. C., & Bouton, M. E. (2019). Resurgence in humans: Reducing relapse by increasing generalization between treatment and testing. Journal of Experimental Psychology: Animal Learning and Cognition, 45(3), 338–349. https://doi.org/10.1037/xan0000209
Tiger, J. H., Hanley, G. P., & Bruzek, J. (2008). Functional communication training: A review and practical guide. Behavior Analysis in Practice, 1(1), 16-23. https://doi.org/10.1007/BF03391716
Trask, S. (2019). Cues associated with alternative reinforcement during extinction can attenuate resurgence of an extinguished instrumental response. Learning & Behavior, 47(1), 66–79. https://doi.org/10.3758/s13420-018-0339-9
Trask, S., & Bouton, M. E. (2016). Discriminative properties of the reinforcer can be used to attenuate the renewal of extinguished operant behavior. Learning & Behavior, 44(2), 151–161. https://doi.org/10.3758/s13420-015-0195-9
Trask, S., Keim, C. L., & Bouton, M. E. (2018). Factors that encourage generalization from extinction to test reduce resurgence of an extinguished operant response. Journal of the Experimental Analysis of Behavior, 110(1), 11–23. https://doi.org/10.1002/jeab.446
Volkert, V. M., Lerman, D. C., Call, N. A., & Trosclair-Lasserre, N. (2009). An evaluation of resurgence during treatment with functional communication training. Journal of Applied Behavior Analysis, 42(1), 145–160. https://doi.org/10.1901/jaba.2009.42-145
Wathen, S. N., & Podlesnik, C. A. (2018). Laboratory models of treatment relapse and mitigation techniques. Behavior Analysis: Research and Practice, 18(4), 362-387. https://doi.org/10.1037/bar0000119
Willcocks, A. L., & McNally, G. P. (2014). An extinction retrieval cue attenuates renewal but not reacquisition of alcohol seeking. Behavioral Neuroscience, 128(1), 83–91. https://doi.org/10.1037/a0035595
Williams, B. A. (1983). Another look at contrast in multiple schedules. Journal of the Experimental Analysis of Behavior, 39(2), 345–384. https://doi.org/10.1901/jeab.1983.39-345
Winterbauer, N. E., & Bouton, M. E. (2010). Mechanisms of resurgence of an extinguished instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes, 36(3), 343–353. https://doi.org/10.1037/a0017365
Author note
This research fulfilled partial requirements of the first author’s Doctor of Philosophy degree from Utah State University. This work was funded in part by Grant R01HD093734 (T.A.S.) from the Eunice K. Shriver National Institute of Child Health and Human Development and Utah State University’s College of Education and Human Services Graduate Student Research Award. The authors would like to thank Gregory Madden, Katherine Brown, Timothy Slocum, and Michael Twohig for advising on this research. K.O.B. is now at the Vermont Center on Behavior and Health at the University of Vermont.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Browning, K.O., Shahan, T.A. Examination of alternative-response discrimination training and resurgence in rats. Learn Behav 49, 379–396 (2021). https://doi.org/10.3758/s13420-021-00470-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-021-00470-9