Introduction
Establishment and impacts of invasive species depend on their biological attributes, their biotic interactions with the native community, and the environmental characteristics of the invaded ecosystem (Keane and Crawley Reference Keane and Crawley2002; Lloret et al. Reference Lloret, Médail, Brundu, Camarda, Moragues, Rita and Hulme2005; Pysek et al. Reference Pyšek, Prach, Šmilauer, Pyšek, Prach, Rejmánek and Wade1995). Introduced plants may become aggressive invaders outside their home ranges for a number of reasons, including release from natural enemies, higher performance in a new site, direct chemical (allelopathic) interference with native plant performance, and variability in the responses and resistance of native systems to invasion (Blossey and Nötzold Reference Blossey and Nötzold1995; Callaway and Aschehoug Reference Callaway and Aschehoug2000). Introduced plant species might be released from constraints present in native environments, allowing individuals of a species in an alien environment to be taller and more vigorous and to produce more seeds (Blossey Reference Blossey1999; Schmidt et al. Reference Schmidt, Hickman, Channell, Harmoney and Stark2008).
Another major explanation for the success of invading species is that the invader possesses individual traits or a combination of traits that are unique or underrepresented in the recipient community, allowing the invader to exploit resources or opportunities not exploited by the native community (Fargione et al. Reference Fargione, Brown and Tilman2003; Vitousek et al. Reference Vitousek, Walker, Whiteaker, Mueller Dombois and Matson1987). Studies have generally found that invasive plant species have higher relative growth rates, greater leaf-area ratios, and maximal photosynthetic rates compared with natives plant species (Grotkopp et al. Reference Grotkopp, Rejmánek and Rost2002; McDowell Reference McDowell2002).
One mechanism that may facilitate invasion by introduced plant species is increased resource availability resulting from disturbance or low resource uptake by the native plant community. Habitats with increased light and nutrients tend to be more productive for invasives (which are often disturbance specialists), which leads to higher growth rates and higher rates of spread (Meekins et al. Reference Meekins, Ballard and McCarthy2001). Phenotypic plasticity may help invasive plants to exploit a wider range of environmental conditions than native species (Hulme Reference Hulme2008; Lande Reference Lande2009; Sultan Reference Sultan2000). Invasive plant species often have more efficient water use, better nitrogen uptake, and higher biomass and can produce seeds that are more likely to germinate compared with natives (DeFalco et al. Reference DeFalco, Bryla, Smith-Longozo and Nowak2003). There is evidence that some invasive Rubus are able to rapidly and efficiently exploit a wide range of soil moisture conditions (Caplan and Yeakley Reference Caplan and Yeakley2010).
Management Implications
Rubus niveus (blackberry) is commonly controlled in the Galapagos using chemicals to kill adult plants. While effective in the short term, this creates gaps that may stimulate the reinvasion of R. niveus from the seedbank. Moreover, the long-term effects of herbicide use on non-target species may slow down the regeneration of native plant communities, giving a competitive advantage to R. niveus. This study provides evidence of the need of two management actions in addition to chemical control of adult plants to allow native Scalesia forest to recuperate. First, a mechanism to reduce the size of the R. niveus seedbank is critical to reduce reinvasion after chemical control of adult plants, and second, active high-density planting using young plants of fast-growing species such as Scalesia pedunculata and Tournefortia rufo-sericea can help by shading bare soil, reducing the germination rate of R. niveus from the seedbank.
Finally, the success of many plant species as invaders is increased by their capacity to maintain persistent stores of seeds in the soil (Passos et al. Reference Passos, Marchante, Pinho and Marchante2017; Richardson and Kluge Reference Richardson and Kluge2008). The ability to produce a large number of seeds together with high germination rates increases invasion success (Greenberg et al. Reference Greenberg, Crownover and Gordon1997; Rejmánek and Richardson Reference Rejmánek and Richardson1996). The more propagules an organism produces, the greater its chances of becoming established (Williamson Reference Williamson1996). However, while the importance of traits to attributes of invasive success is recognized, it is now generally accepted that many other factors, such as the biological and ecological interactions with other species in the new environment, play an equally important role (Dunbar and Facelli Reference Dunbar and Facelli1999).
Invasive blackberry (Rubus niveus Thunb.) is a thorny, perennial shrub native to India, southeastern Asia, the Philippines, and Indonesia (Morton Reference Morton and Morton1987). This species has a wide climatic range, from near sea level to montane environments at 3,000 m. However, R. niveus is not resistant to drought or frost (Morton Reference Morton and Morton1987; Wagner et al. Reference Wagner, Herbst and Sohmer1999). In places with harsh winters, the species behaves as an annual plant growing back each spring from the seedbank or roots, while in most tropical areas it can grow all year round (Morton Reference Morton and Morton1987). The plant is cultivated throughout the world for its heavy production of sweet fruit. Rubus niveus has been introduced into Central America, South America, the United States, South Africa, and Australia (Morton Reference Morton and Morton1987; St. Quinton et al. Reference Quinton, Fay, Ingrouille and Faull2011).
In the Galapagos, R. niveus has invaded grass- and farmlands, shrublands, and forest alike. It forms dense thickets, replacing native vegetation and threatening several native communities, including the Scalesia [Scalesia pedunculata Hook.f. (Asteraceae)] forest (Itow Reference Itow2003; Renteria and Buddenhagen Reference Renteria and Buddenhagen2006; Wilkinson et al. Reference Wilkinson, Naeth and Schmiegelow2005). On Santa Cruz Island, the Scalesia forest is situated within the humid zone (600 m asl) and receives a mean annual precipitation of approximately 1,845 mm (Itow and Mueller Dombois Reference Itow and Mueller Dombois1992). Soils are up to 1-m deep, of basaltic origin, well weathered, and sandy loam in texture (Laruelle Reference Laruelle1966; Stoops Reference Stoops2014). The humid zone comprises a number of subzones that vary between islands and include the Scalesia Zone, Miconia Zone, and Fern-Sedge Zone (Stoops Reference Stoops2014; Tye Reference Tye2006). The Scalesia Zone is the most fertile habitat in the archipelago, where agricultural settlements were established (Moll Reference Moll1990). In Santa Cruz Island, this zone was historically dominated by the endemic tree S. pedunculata; however, land-use change has severely reduced the original vegetation, leaving small and sparse fragments (Mauchamp and Atkinson Reference Mauchamp and Atkinson2011; Moll Reference Moll1990; Watson et al. Reference Watson, Trueman, Tufet, Henderson and Atkinson2010). The Scalesia forest at Los Gemelos on Santa Cruz Island is one of the best remnants of this humid vegetation type. This forest is still dominated by S. pedunculata and constitutes the habitat of many endemic and native species (Hamann Reference Hamann2001; Itow Reference Itow1995). The Scalesia forest has been invaded by a number of introduced plant species, including R. niveus (Renteria and Buddenhagen Reference Renteria and Buddenhagen2006).
Several life-history traits may contribute to R. niveus invasiveness in the Scalesia forest. As is the case for other invasive Rubus spp., rapid growth of the shoot and root, ability to reproduce vegetatively, early flowering, self-compatibility, and high rate of seed production may contribute to its success in the Galapagos Islands (Baret et al. Reference Baret, Cournac, Thébaud, Edwards and Strasberg2008; Gerrish et al. Reference Gerrish, Stemmermann and Gardner1992). A possible mechanism that may enable R. niveus and other invasive species to successfully invade and persist in a wide range of habitats is superior competitive ability over native species as measured by its rapid growth, early maturity, large quantities of seeds and fruit, effective seed dispersal, vegetative reproduction, and generation of dense shade (Atkinson et al. Reference Atkinson, Rentería and Simbaña2008; Bellingham et al. Reference Bellingham, Duncan, Lee and Buxton2004; Landázuri 2002; Rejmánek and Richardson Reference Rejmánek and Richardson1996).
Because invasive species represent a threat to native biota and contribute to the decrease of native biological diversity (Levine et al. Reference Levine, Antonio, Dukes, Grigulis and Lavorel2003; Mooney and Cleland Reference Mooney and Cleland2001), it is fundamental to identify the mechanisms, traits, or external factors that contribute to successful invasion (Lake and Leishman Reference Lake and Leishman2004; Pyšek et al. Reference Pyšek, Richardson, Rejmánek, Webster, Williamson and Kirschner2004). Understanding interactions between invaders and residents and the mechanisms by which invasive species outperform native species is essential to efficient management and restoration of native-dominated habitats (Richardson and Kluge Reference Richardson and Kluge2008). Management of invasive plants in natural areas should aim at a self-sustaining ecosystem with desired species composition and ecosystem functions (D’Antonio et al. Reference D’Antonio, August-Schmidt, Fernandez-Going, Palmer, Zedler and Falk2016).
This study examines the growth performance of R. niveus relative to the four most common native plant species from the Scalesia forest in Santa Cruz Island in the Galapagos Islands: the tree S. pedunculata, the woody vine-shrub Chiococca alba (L.) Hitchc. (Rubiaceae), and the shrubs Psychotria rufipes Hook.f. (Rubiaceae) and Tournefortia rufo-sericea Hook.f. (Boraginaceae). We used greenhouse experiments to compare: (1) the relative growth rate of R. niveus and these native species and (2) the tolerance and performance of both native species and R. niveus under different stress conditions. Additionally, experimental plots were established in the Scalesia forest to assess interspecific competition in infested areas, as well as seedbanks and seedling recruitment from R. niveus.
Material and Methods
Relative Growth Rate
To assess the relative growth rate of R. niveus and the four native species, seedlings of each species were collected from the Scalesia forest (approximately 2- to 3-wk old, 5 cm average height) and grown in individual plastic pots (1,500 cm3) containing soil from the highland farms (agricultural areas adjacent to the Scalesia forest) under a shade house (70% of natural sunlight, with a 12-h daylight regime, average annual temperature of 22.2 C). Fifteen seedlings of each species were randomly positioned in the shade house and watered as required. Seedlings dying within 2 wk after potting were replaced. Initial and final stem length and cover area index (represented by the product of minimum and maximum length) of each plant were measured. After 8 mo, all plants were harvested and samples were dried at 45 C and weighed to determine foliar and root biomass. We use linear models (multivariate analysis of variance [MANOVA]) with species as fixed effects to determine significant differences in relative growth and biomass production between species; values of each response variable were log transformed. The statistical analysis for the whole study was performed using the computing environment R (R Core Team 2018), an open-access software environment.
Stress Tolerance
To determine the response of R. niveus and the four native species to different light and water conditions, seedlings of the different species were planted in individual plastic pots (1,500 cm3) containing soil from the highland farms and grown under two different light levels (90% and ∼10% of ambient sunlight). These represent an approximation for “open ground” and “closed canopy” sunlight conditions at the Scalesia forest; the low-sunlight treatment was provided using wooden frames covered with shade cloth. Two different watering regimens were used (500 ml and 250 ml as proxies of 100% and 50% volume saturation, respectively), water was applied manually twice a week. A two-way factorial design was used with 12 replicates per treatment (each plant as a replicate). Initial and final stem diameter and length per plant were measured. After 5 mo, the experiment was stopped; plants were harvested, dried at 45 C, and weighed to determine foliar and root biomass. We used a linear model (MANOVA) to assess the influence of light and water treatments as fixed effects on the variation in relative growth and biomass production of the different species; values of each response variable were log transformed.
Seedbank and Competition between Recruits
To assess the seedbank contribution to the regeneration process of the Scalesia forest, a set of 20 paired plots (4 by 4 m) were set up along the Scalesia forest. Each pair consisted of heavily invaded (“invaded areas,” with at least 90% R. niveus cover) and a nearby uninvaded plot (“uninvaded vegetation”). The uninvaded plots were selected to represent as closely as possible the same habitat conditions as the corresponding invaded plots. The vegetation of heavily invaded plots was cleared completely (using a machete and spraying R. niveus stems with glyphosate (Roundup 2%, Roundup Custom, Bayer CropScience LLC, Research Triangle Park, NC, USA), as suggested by Renteria et al. [Reference Renteria, Atkinson, Guerrero, Mader, Soria and Taylor2006]) to assess competition recruits. In each plot, five soil samples were taken within centrally located 1-m2 subplots using a metal core (4.5-cm diameter by 5-cm deep). Soil cores from each plot were mixed together and spread out into plastic trays containing a layer of sterile vermiculite; trays were watered regularly and kept in a shade house to monitor germination over 5 mo. Seedling emergence was recorded as counts per species and then removed after identification to avoid double counting. Regeneration of vegetation in the invaded plots 9 mo after clearing was assessed by estimating the species cover percentage within the 1-m2 subplots. Generalized linear models (GLM) with Poisson distribution were used to compare variation of seedbank composition among “invaded” and “uninvaded” treatments, with these treatments as fixed factors; germination values were log transformed.
Results and Discussion
Relative Growth Rate
The percentage of survivorship of most of the species was 100%, except for S. pedunculata (87%). Growth parameters differed significantly among species, expressed both as increased length and cover (Figure 1A) and final foliar and root dry biomass (Figure 1B). Rubus niveus showed greater increase in length than other species (F(4, 68) = 69.3, P < 0.001). Scalesia pedunculata had the greatest incremental growth for stem length among the native species. Scalesia pedunculata, T. rufo-sericea, and R. niveus showed a significantly higher foliar cover increase than the woody vine C. alba and the endemic shrub P. rufipes (F(4, 68) = 18.8, P < 0.001). Rubus niveus showed greater production of foliar biomass compared with all native species tested, (F(4, 68) = 7.7, P < 0.001). Rubus niveus and T. rufo-sericea produced greater root biomass (F(4, 68) = 28.6, P < 0.001).
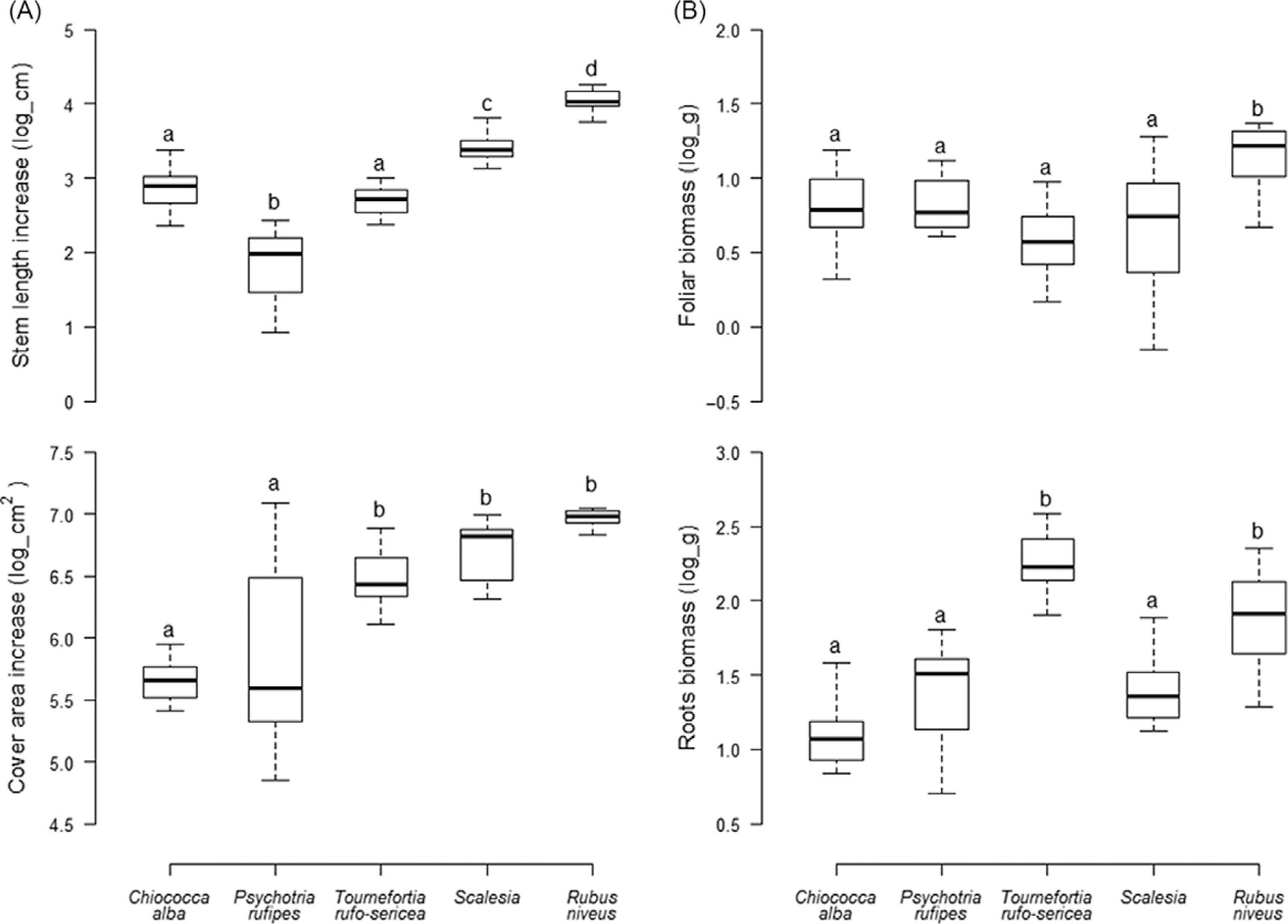
Figure 1. Final (A) stem length and foliar cover increase and (B) foliar and root dry biomass values of Rubus niveus and four native species of the Scalesia forest. Median value of the growth parameters; letters denote significant differences among species (P < 0.05).
Overall, R. niveus grew faster relative to the native species. After 8 mo, R. niveus showed greater increase in size and biomass production than native species for each of the four growth parameters. Among the native species, S. pedunculata and T. rufo-sericea showed greater growth, whereas C. alba and P. rufipes showed the lowest values of growth rate and biomass production. On average, R. niveus increase was: 8.5- and 3.5-fold greater than P. rufipes and T. rufo-sericea in stem length; 4- and 2.5-fold greater than C. alba and P. rufipes in foliar cover; 1.7- and 1.6-fold greater than T. rufo-sericea and S. pedunculata in foliar biomass; and 2.3- and 1.7-fold greater in root biomass.
Rubus niveus showed higher growth rates than the native species, supporting the hypothesis that invasive plant species generally have a performance advantage over native species (Daehler Reference Daehler2003; Milberg et al. Reference Milberg, Lamont and Perez1999; Rejmánek and Richardson Reference Rejmánek and Richardson1996). Under the same environmental conditions, R. niveus showed faster growth rates for stem length and cover area and higher production of foliar and root biomass compared with the four native species. Rapid growth may enable R. niveus to quickly occupy free space and therefore outgrow associated native shrub and tree species (McDowell and Turner Reference McDowell and Turner2002; Pysek et al. Reference Pyšek, Prach, Šmilauer, Pyšek, Prach, Rejmánek and Wade1995; Williamson and Fitter Reference Williamson and Fitter1996); however, it is not known whether this differential growth rate continues through to maturity. In general, native woody species allocate resources to develop stems and branches (Grime Reference Grime2001); the high production of foliar and root biomass may give R. niveus an advantage over native ecologically similar species when accessing resources such as water, nutrients, and light (Grotkopp et al. Reference Grotkopp, Rejmánek and Rost2002; Kolar and Lodge Reference Kolar and Lodge2001).
Stress Tolerance
Survivorship was greater than 90% for most species within all treatment types. However, the endemic tree S. pedunculata exhibited 100% mortality within the shaded treatment; therefore, this species was removed from the analysis. Light availability significantly affected the growth parameters (stem diameter, stem length, and foliar and root biomass) of both R. niveus and the three native species, whereas watering regimens only affected the foliar and root biomass of all species. The interaction of light and water treatment did not significantly affect growth parameters (Figure 2; Table 1).

Figure 2. Effects of light and water availability on (A) the relative diameter and length growth and (B) final foliar and root biomass of Rubus niveus and three native species of the Scalesia forest. Median value of the growth parameters.
Table 1. Multivariate analysis of variance (MANOVA) results for the growth parameters (stem diameter, stem length, and foliar and root biomass) of Rubus niveus and three native species of the Scalesia forest under different light and water availability.

All species in the stress experiment, except for S. pedunculata, showed high survival (>90%) under the different light and water treatments. Almost all S. pedunculata plants died in shaded treatments, showing a limited tolerance to low light conditions. Scalesia pedunculata is an heliophytic successional tree whose regeneration depends on canopy openings (Hamann Reference Hamann1979). Light was the major cause of variation in growth and biomass production of both R. niveus and native species. Because the humid highland vegetation of the Galapagos Island has evolved with highly variable rainfall conditions—high precipitation during extreme El Niño events often followed by marked droughts (La Niña) (Hamann Reference Hamann and Robinson1985; Wilkinson et al. Reference Wilkinson, Naeth and Schmiegelow2005)—native species are adapted to survive prolonged hot-season droughts. Conversely, R. niveus was the only species clearly affected by water stress. Studies have shown that abundant light (Baret et al. Reference Baret, Cournac, Thébaud, Edwards and Strasberg2008) and water access are fundamental factors for the successful invasion of other Rubus spp. (Caplan and Yeakley Reference Caplan and Yeakley2010). Rubus niveus experienced higher sensitivity than native species to light and water conditions expressed by higher variation in growth and biomass production. This trait may help R. niveus to more readily access resources than slower growing native species (Vasquez et al. Reference Vasquez, James, Monaco and Cummings2010), especially once resources become available after disturbance (Funk and Vitousek Reference Funk and Vitousek2007; King and Grace Reference King and Grace2000). Natural disturbance is a critical element for the regeneration of Scalesia forest in Galapagos (Itow and Mueller Dombois Reference Itow and Mueller Dombois1992; Wilkinson et al. Reference Wilkinson, Naeth and Schmiegelow2005); disturbance caused by tree fall leads to openings in the forest canopy, allowing S. pedunculata recruitment (Vasquez et al. Reference Vasquez, James, Monaco and Cummings2010; Wilkinson et al. Reference Wilkinson, Naeth and Schmiegelow2005). However, R. niveus is now filling these gaps and is more competitive than S. pedunculata (Renteria et al. Reference Renteria, Atkinson, Crespo and Gardener2021).
In communities with seasonally fluctuating resource regimes such as the Scalesia forest, an invasive plant could potentially have an advantage by exploiting surplus resources during periods of high availability (Caplan and Yeakley Reference Caplan and Yeakley2010; Funk and Vitousek Reference Funk and Vitousek2007). This may be the case for R. niveus, which showed more sensitivity to light and water availability; this particular trait may allow R. niveus to more effectively exploit resources or opportunities not utilized by the native species (Davis and Thompson Reference Davis and Thompson2000; Meiners Reference Meiners2007). Although all habitats are vulnerable to invasion (Williamson Reference Williamson1996), the results from this experiment indicate water availability is a limiting factor for the distribution of R. niveus. The species has only been reported from the humid zone of the Galapagos highlands, unlike the four native species, which are more widespread (Atkinson et al. Reference Atkinson, Rentería and Simbaña2008; Renteria and Buddenhagen Reference Renteria and Buddenhagen2006). Rubus niveus distribution seems to be limited to the humid and very humid zones, where edaphic conditions (especially depth, moisture-holding capacity, and fertility) may be more suitable (Atkinson et al. Reference Atkinson, Rentería and Simbaña2008; Hamann Reference Hamann2001; Itow Reference Itow1995).
Seedbank and Competition between Recruits
A total of 1,171 seedlings of R. niveus, 960 seedlings of native species, and 41 seedlings of other introduced species emerged from soil samples within invaded areas, whereas 1,648 seedlings of native species, 57 seedlings of R. niveus, and 12 seedlings of other introduced species emerged from soil samples from the uninvaded areas. A total of 22 vascular plant species were recorded, comprising 17 native and 5 introduced species. Species consisted of 15 herbs (3 introduced), 1 vine, 3 shrubs (1 introduced), and 3 trees (1 introduced); 82% of the total species occurred within uninvaded areas, while 64 % of the species occurred in invaded areas, but this difference was not significant.
There was a significant difference between the number of R. niveus seedlings and the number of native species seedlings that emerged from soil samples collected within invaded areas (GLM, quasi-Poisson error distribution: df = 38, t = −3.622, P < 0.001). Seedlings of R. niveus also emerged from soil samples collected from uninvaded areas; however, the number was significantly lower compared with the number of seedlings of native species (GLM, quasi-Poisson error distribution: df = 38, t = 4.178, P < 0.001) (Figure 3).

Figure 3. Density (median values) of emerged seedlings of Rubus niveus and native species geminated from the soil samples in invaded and uninvaded areas. Letters denote significant differences between R. niveus and native species (P < 0.05).
Herbaceous species strongly dominated the soil seedbank in both invaded and uninvaded areas, making up about 76% and 85% of the total number of seedlings, whereas shrubs and trees made up only 15% and 24%, respectively. Seed germination from soil samples from invaded areas (Figure 3) is expressed as number of seedlings per square meter and is summarized as follows: R. niveus = 1,200 ± 378.7, herbs = 266.2 ± 63.2, shrubs = 42.5 ± 15.5, and tree species = 43.7 ± 11.3. The number of R. niveus seedlings per square meter that germinated from soil seedbank in invaded areas was 4.5-, 28.2-, and 27.5-fold greater than the number of native herbs, shrubs, and trees, respectively. Rubus niveus seedlings emerged more rapidly than native species seedlings. Within the 5 wk, more than 70% of the total seedlings of R. niveus had emerged compared with only 40% of the native species seedlings.
In invaded areas, the total number of emerged seedlings of R. niveus was greater than for native species, particularly when compared with the number of seedlings from woody shrubs and trees species. This may result in a competitive advantage for R. niveus during the regeneration process; therefore, managed sites can be reinvaded easily after control is carried out (Bekker et al. Reference Bekker, Verweij, Smith, Reine, Bakker and Schneider1997; D’Antonio and Meyerson Reference D’Antonio and Meyerson2002; Oke et al. Reference Oke, Oladipo, Ndiribe, Akinyemi and Ojo2009; Renteria et al. Reference Renteria, Gardener, Panetta and Crawley2012b; Vilà and Gimeno Reference Vilà and Gimeno2007). Invasive plant species often produce an abundance of seeds and have very large persistent seedbanks (Lonsdale Reference Lonsdale1988; Oke et al. Reference Oke, Oladipo, Ndiribe, Akinyemi and Ojo2009). In the Galapagos Islands, R. niveus fruits all year round, producing copious quantities of seed and forming a large seedbank with up to 7,000 seeds m−2 (Landázuri 2002). In addition to its large seedbank, more rapid germination compared with native species delivers a competitive advantage for R. niveus in terms of resources and space occupancy (Perez et al. Reference Perez, Lamont, Marwick and Lamont2000).
As expected, shrubs and trees of native species were poorly represented, whereas herbaceous species were the most predominant group; similar results were found by Wilkinson (Reference Wilkinson2002) when comparing the soil seedbank of the Scalesia forest with an abandoned pasture. Although the stand vegetation is dominated by the endemic tree S. pedunculata and shrubs species such as T. rufo-sericea and C. alba (Shimizu Reference Shimizu1997), presence of these species was not evident in the seedbank. Invaded areas and uninvaded areas showed similar native species seedbank; this indicates that native species are not yet limited in invaded areas, offering potential for regeneration if the invader can be controlled or even eliminated (Dunbar and Facelli Reference Dunbar and Facelli1999; Panetta Reference Panetta1982; Richardson et al. Reference Richardson, Macdonald and Forsyth1989; Turner et al. Reference Turner, Scott and Spafford2008).
The seedbank and standing vegetation after control of R. niveus had 16 species in common. These represented 73% of all species in the seedbank and 52% of the species in the standing vegetation. This variation was the result of a difference in the number of species: 22 species in the seedbank compared with 31 species in the standing vegetation. There was no consistent relationship between the number seedlings per square meter available in the soil seedbank and the aboveground cover regenerated after control of R. niveus (Figure 4). Although the number of R. niveus seeds available in the soil was considerably greater than the seed counts for all other growth forms, at 9 mo after control, herb and tree layer showed greater values in ground cover than R. niveus.

Figure 4. Relationship between the density of seedlings per square meter and the aboveground cover after Rubus niveus control in invaded areas. Values represent the averages of number of seedlings geminated from soil samples and averages of vegetation cover within 1-m2 subplots. Bar heights represent mean values, and error bars represent ±SE.
There was a low correspondence between the available seedbank and the regenerated vegetation after control of R. niveus. Although there was a large quantity of R. niveus seedlings germinating from the soil samples, at 9 mo after control, the regeneration of R. niveus on the experimental plots was very low. The lack of soil moisture due to a severe drought that affected the archipelago during 2009 may have affected the regeneration of R. niveus. This might be the reason why the seedbank did not reflect the standing vegetation cover. As demonstrated with the soil sample germination experiment, R. niveus seeds have the ability to germinate faster than native species under ideal soil moisture conditions. This has been evident in areas under intensive management where reinvasion from the seedbank has occurred right after adult plants have been controlled (Landázuri 2002; Renteria et al. Reference Renteria, Gardener, Panetta and Crawley2012b). On the other hand, regeneration of native species, particularly herbs and trees, demonstrated the greater tolerance of native species to water stress conditions (Hamann Reference Hamann1981; Shimizu Reference Shimizu1997).
Rubus niveus and natives showed tolerance to changes in light and water conditions; however, R. niveus performed better than native species when both moisture and light were in ample supply. The soil seedbank data demonstrated that native species may not be seed limited in the invaded areas; however, the enormous seedbank of R. niveus suggests this could constrain the restoration of the native diversity from the native soil seedbank alone. This study is based upon work carried out on seedlings under controlled conditions for a limited period of time. As such, it cannot address the total complexity of the R. niveus invasion within the Scalesia forest, although the findings tend to conform to established invasive plant theories.
Management actions for the restoration of native communities should encourage resistance to reinvasion of R. niveus in order to favor the persistence or recovery of desirable species (D’Antonio et al. Reference D’Antonio, August-Schmidt, Fernandez-Going, Palmer, Zedler and Falk2016). Chemical control can be an effective short-term management intervention to reduce R. niveus invasion; however, there is always a risk of reinvasion from the seedbank resulting in the need for ongoing treatment. Complementary activities should be considered for integrated management and restoration of the Scalesia forest, such as the reduction of the prolific seedbank and active establishment of native canopy species. Certain native species could act as a natural barrier to stop colonization and expansion of undesirable plant species (Kettenring and Adams Reference Kettenring and Adams2011; Shafroth et al. Reference Shafroth, Cleverly, Dudley, Taylor, Van Riper, Weeks and Stuart2005). The use of fast-growing native species with a high reproductive rates and adapted to the site conditions should be considered; young plants of S. pedunculata and T. rufo-sericea planted at high densities could provide a rapid source of shade early in the season and limit resources to reduce the reestablishment of R. niveus from the seedbank.
The use of biological control could be a cost-effective long-term control strategy to reduce the density of R. niveus to below a threshold of impact (Renteria et al. Reference Renteria, Gardener, Panetta, Atkinson and Crawley2012a). While there are some risks involved with the introduction and release of any new exotic organism to the archipelago, these risks are almost negligible if the protocols to develop a biological agent are strictly followed. Furthermore, there are no Rubus or Rosaceae native to the Galapagos. In addition, R. niveus is part of a tribe of Rubus species from the Old World, so it is very unlikely that a biological control agent would affect the New World Rubus species native to mainland Ecuador.
Acknowledgments
The Galapagos Conservation Trust provided a PhD scholarship to JLR and the Charles Darwin Foundation and the Rufford Small Grants Foundation provided funds to cover fieldwork costs. We thank the Galapagos National Park Directorate for the permit to carry out this study. We are grateful for the contributions made by Reitumetse Molotsane to earlier versions of this article. We also thank Cristina Banks, Bernardo Carreras, and Ana Bento, who provided assistance with graphics and data analyses. No conflicts of interest have been declared.









