Abstract
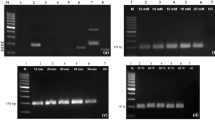
Foot rot caused by Phytophthora is one of the major diseases of black pepper (Piper nigrum L.). Accurate and timely diagnosis of the disease is crucial for its successful management. Although PCR and qPCR assays are used for detection, the cost and time required to perform these assays are high. Recombinase polymerase amplification (RPA) assay has the advantage of minimal assay time and it is performed under isothermal conditions. Hence, RPA assay was developed for the detection of P. capsici and P. tropicalis and compared with newly developed end-point PCR test. Out of three sets of primers analyzed, a primer set based on the Ypt1 gene successfully amplified a 230/231 bp product. Optimum amplification of RPA products were observed when the assay was performed at 37 °C with 14 mM magnesium acetate for 40 min. Sensitivity analysis using serial dilutions indicated that RPA is 10 times more sensitive than end-point PCR. During specificity analysis, non-specific bands were observed with other Phytophthora species, and hence the assay was further refined with betaine wherein addition of 1.0 M betaine avoided amplification of non-specific bands. The optimized RPA assay could detect Phytophthora from infected black pepper leaf, stem and root using both purified DNA and crude extracts. The end-point PCR test successfully differentiated the two species of Phytophthora in a validation test. These results indicate the robustness of the developed end-point PCR and RPA assays and its potential application in detection and differentiation of P. capsici and P. tropicalis infecting black pepper.






Similar content being viewed by others
References
Ahmed, F. A., Larrea-Sarmiento, A., Alvarez, A. M., & Arif, M. (2018). Genome informed diagnostics for specific and rapid detection of Pectobacterium species using recombinase polymerase amplification coupled with a lateral flow device. Scientific Reports, 8, 15972.
Anandaraj, M., & Sarma, Y. R. (1990). A simple baiting technique to detect and isolate Phytophthora capsici (‘P. palmivora’ MF4) from soil. Mycological Research, 94, 1003–1004.
Babu, B., Ochoa-Coronac, F. M., & Pareta, M. L. (2018). Recombinase polymerase amplification applied to plant virus detection and potential implications. Analytical Biochemistry, 546, 72–77.
Blair, J. E., Coffey, M. D., & Martin, F. N. (2012). Species tree estimation for the late blight pathogen, Phytophthora infestans and close relatives. PLoS One, 7, e37003.
Craw, P., & Balachandran, W. (2012). Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab on a Chip, 12, 2469–2486.
Dai, T., Yang, X., Hu, T., Jiao, B., Xu, Y., Zheng, X., & Shen, D. (2019). Comparative evaluation of a novel recombinase polymerase amplification-lateral flow dipstick (RPA-LFD) assay, LAMP, conventional PCR and leaf-disc baiting methods for detection of Phytophthora sojae. Frontiers in Microbiology, 10, 1884.
Erwin, D. C., & Ribeiro, O. K. (1996). Phytophthora diseases worldwide. St. Paul: American Phytopathological Society Press.
Henke, W., Herdel, K., Jung, K., Schnorr, D., & Loening, S. A. (1997). Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Research, 25, 3957–3958.
Kapoor, R., Srivastava, N., Kumar, S., Saritha, R. K., Sharma, S. K., Jain, R. K., & Baranwal, V. K. (2017). Development of a recombinase polymerase amplification assay for the diagnosis of banana bunchy top virus in different banana cultivars. Archives of Virology, 162, 2791–2796.
Kox, L. F. F., Brouwershaven, I. V., Vossenberg, B. V. D., Beld, H. V. D., Bonants, P. J. M., & Gruyter, J. D. (2007). Diagnostic values and utility of immunological, morphological, and molecular methods for in planta detection of Phytophthora ramorum. Phytopathology, 97, 1119–1129.
Li, J., Macdonald, J., & Stetten, F. (2019). Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst, 144, 31–67.
Londoño, M. A., Harmon, C. L., & Polston, J. E. (2016). Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virology Journal, 13, 48.
Luo, G.-C., Yi, T.-T., Jiang, B., Guo, X.-l., & Zhang, G.-Y. (2019). Betaine-assisted recombinase polymerase assay with enhanced specificity. Analytical Biochemistry, 575, 36–39.
Martin, F. N., Abad, Z. G., Balci, Y., & Ivors, K. (2012). Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Disease, 96, 1080–1103.
Miles, T. D., Martin, F. N., & Coffey, M. D. (2015). Development of rapid isothermal amplification assays for detection of Phytophthora spp. in plant tissue. Phytopathology, 105, 265–278.
Mok, E., Wee, E., Wang, Y., & Trau, M. (2016). Comprehensive evaluation of molecular enhancers of the isothermal exponential amplification reaction. Scientific Reports, 6, 37837.
Munawar, M., Toljamo, A., Martin, F., & Kokko, H. (2019). Recombinase polymerase amplification assay for fast, sensitive and on-site detection of Phytophthora cactorum without DNA extraction. European Journal of Horticultural Science, 84, 14–19.
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., & Hase, T. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Research, 28, E63.
Pandian, R. T. P., Bhat, A. I., Biju, C. N., & Sasi, S. (2018). Development of diagnostic assays for rapid and sensitive detection of Phytophthora infecting major spices and plantation crops. Journal of Spices and Aromatic Crops, 27, 119–130.
Rojas, J. A., Miles, T. D., Coffey, M. D., Martin, F. N., & Chilvers, M. I. (2017). Development and application of qPCR and RPA genus- and species-specific detection of Phytophthora sojae and P. sansomeana root rot pathogens of soybean. Plant Disease, 101, 1171.
Sarma, Y. R., Anandaraj, M., & Venugopal, M. N. (1997). Phytophthora foot rot of black pepper. In: Agnihotri, V.P., Sarbhoy, A.K., Singh, D.V. (Eds.), Management of Threatening Diseases of National Importance. Malhotra Publishing House, New Delhi, pp. 237–248.
Schrader, C., Schielke, A., Ellerbroek, L., & Johne, R. (2012). PCR inhibitors–occurrence, properties and removal. Journal of Applied Microbiology, 113, 1014–1026.
Sheji, C., Renu, S. G., Balaji, S., & Anandaraj, M. (2009). Ribosomal DNA analysis of three Phytophthora species occurring in India. Indian Phytopathology, 62, 155–162.
Si Ammour, M., Bilodeau, G. J., Tremblay, D. M., Heyden, H. V., Yaseen, T., Varvaro, L., & Carisse, O. (2017). Development of real-time isothermal amplification assays for on-site detection of Phytophthora infestans in potato leaves. Plant Disease, 101, 1269–1277.
Silvar, C., Duncan, J. M., Cooke, D. E. L., Williams, N. A., Díaz, J., & Merino, F. (2005). Development of specific PCR primers for identification and detection of Phytophthora capsici Leon. European Journal of Plant Pathology, 112, 43–52.
Tooley, P. W., Bunyard, B. A., Carras, M. M., & Hatziloukas, E. (1997). Development of PCR primers from internal transcribed spacer region 2 for detection of Phytophthora species infecting potatoes. Applied and Environmental Microbiology, 63, 1467–1475.
Yu, J., Shen, D., Dai, T., Lu, X., Xu, H., & Dou, D. (2019). Rapid and equipment free detection of Phytophthora capsici using lateral flow strip-based recombinase polymerase amplification assay. Letters in Applied Microbiology, 69, 64–70.
Zhang, Z. G., Li, Y. Q., Fan, H., Wang, Y. C., & Zheng, X. B. (2006). Molecular detection of Phytophthora capsici in infected plant tissues, soil and water. Plant Pathology, 55, 770–775.
Zhao, C., Sun, F., Li, X., Lan, Y., Du, L., Zhou, T., & Zhou, Y. (2019). Reverse transcription-recombinase polymerase amplification combined with lateral flow strip for detection of rice black-streaked dwarf virus in plants. Journal of Virological Methods, 263, 96–100.
Acknowledgments
The authors are thankful to The Director, ICAR-Indian Institute of Spices Research, Kozhikode, Kerala for providing facilities and Indian Council of Agricultural Research, New Delhi for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants and/or animals
Not applicable.
Informed consent
All authors have reviewed the manuscript and approved its submission to the European Journal of Plant Pathology.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jeevalatha, A., Biju, C.N. & Bhai, R.S. Ypt1 gene-based recombinase polymerase amplification assay for Phytophthora capsici and P. tropicalis detection in black pepper. Eur J Plant Pathol 159, 863–875 (2021). https://doi.org/10.1007/s10658-021-02211-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02211-0