Abstract
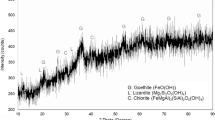
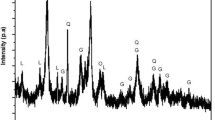
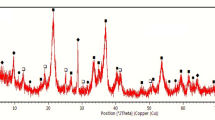
The diminution of sulfide ore deposit as the main source of nickel extraction initiates the development of alternative methods to maximize the processing of low-grade nickel ore including limonitic laterites. Aiming to produce ferronickel with high nickel content and recovery, the study used sodium thiosulfate and natural sulfur as novel additives to be employed in selective reduction techniques. The reduction process was conducted at 1400 °C for 6 h in a coal–limestone bed. Ores and the as-reduced products were characterized by Emission Dispersive X-Ray (EDX) and X-Ray Diffraction (XRD) to determine the elemental composition in the product and examine the phase transformation of ores. Results showed that utilization of 10%wt Na2S2O3 resulted in a product with 14.31% Ni content and 93.22% recovery, while the employment of 10%wt natural sulfur yielded a product with 13.62% Ni content and its recovery reaching 97.91%. Phase identification of slag product using XRD pointed out that both Na2S2O3 and natural sulfur additives assisted the transformation of ore mineralogy in which the kamacite and taenite phases of metal products confirm the successful formation of ferronickel (Fe, Ni). Furthermore, slag products showed a large amount of iron compound, confirming the fact of low iron recovery.
Graphical Abstract














Similar content being viewed by others
References
Marsh E, Anderson E (2011) Ni-Co laterites—a deposit model. US Geol Surv Open File Rep 1259:1–12
Rhamdhani MA, Hayes PC, Jak E (2009) Nickel laterite part 1—Microstructure and phase characterisations during reduction roasting and leaching. Trans Inst Min Metall Sect C Miner Process Extr Metall 118:129–145. https://doi.org/10.1179/174328509X431391
Ashok D, Dalvi W, Gordon B (2004) The past and the future of nickel laterites. In: PDAC 2004 International Convention, Trade Show & Investors Exchange, pp 1–27
Quast K, Connor JN, Skinner W et al (2015) Preconcentration strategies in the processing of nickel laterite ores part 1: Literature review. Miner Eng 79:261–268
Elliott R, Pickles CA, Peacey J (2017) Ferronickel particle formation during the carbothermic reduction of a limonitic laterite ore. Miner Eng 100:166–176. https://doi.org/10.1016/j.mineng.2016.10.020
Whittington BI, Muir D (2000) Pressure acid leaching of nickel laterites: a review. Miner Process Extr Metall Rev 21:527–599. https://doi.org/10.1080/08827500008914177
Mudd GM (2009) Nickel sulfide versus laterite: the hard sustainability challenge remains. In: Proceedings of the 48th Conference of Metallurgists, pp 1–10
Youping Z, Yusheng Z, Zhaoyi L (2008) Technical analysis of producing low Ni pig iron with laterite in a blast furnace. Baosteel Tech Res 36–40
Rhamdhani MA, Hayes PC, Jak E (2009) Nickel laterite part 2—Thermodynamic analysis of phase transformations occurring during reduction roasting. Trans Inst Min Metall Sect C Miner Process Extr Metall 118:146–155. https://doi.org/10.1179/174328509X431409
Rodrigues F, Pickles C, Peacey J et al (2017) Factors affecting the upgrading of a nickeliferous limonitic laterite ore by reduction roasting. Therm Growth Magn Sep Miner 7:176. https://doi.org/10.3390/min7090176
Zhu DQ, Cui Y, Vining K et al (2012) Upgrading low nickel content laterite ores using selective reduction followed by magnetic separation. Int J Miner Process 106–109:1–7. https://doi.org/10.1016/j.minpro.2012.01.003
Li G, Shi T, Rao M et al (2012) Beneficiation of nickeliferous laterite by reduction roasting in the presence of sodium sulfate. Miner Eng 32:19–26. https://doi.org/10.1016/j.mineng.2012.03.012
Pickles CA, Forster J, Elliott R (2014) Thermodynamic analysis of the carbothermic reduction roasting of a nickeliferous limonitic laterite ore. Miner Eng 65:33–40. https://doi.org/10.1016/j.mineng.2014.05.006
Rao M, Li G, Zhang X, Luo J, Peng Z, Jiang T (2016) Reductive roasting of nickel laterite ore with sodium sulfate for Fe-Ni production. Part I: reduction/sulfidation characteristics. Sep Sci Technol 51(8):1408–1420. https://doi.org/10.1080/01496395.2016.1162173
Widyartha B, Setiyorini Y, Abdul F et al (2020) Effective beneficiation of low content nickel ferrous laterite using fluxing agent through Na2SO4 selective reduction. Materwiss Werksttech 51:750–757. https://doi.org/10.1002/mawe.202000007
Cao C, Xue Z, Duan H (2016) Making ferronickel from laterite nickel ore by coal-based self-reduction and high temperature melting process. Int J Nonferrous Metall 05:9–15. https://doi.org/10.4236/ijnm.2016.52002
Zhou S, Wei Y, Li B et al (2016) Mechanism of sodium chloride in promoting reduction of high-magnesium low-nickel oxide ore. Sci Rep. https://doi.org/10.1038/srep29061
Jiang M, Sun T, Liu Z et al (2013) Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. Int J Miner Process 123:32–38. https://doi.org/10.1016/j.minpro.2013.04.005
Abdul F, Pintowantoro S, Purnamasari A (2020) Direct reduction of nickel laterite limonitic ore using a coal-dolomite mixture bed and Na2SO4 as a selective agent. J Chem Technol Metall 55(1):103–110
Pintowantoro S, Abdul F (2019) Selective reduction of laterite nickel ore. Mater Trans 60:2245–2254. https://doi.org/10.2320/matertrans.MT-M2019101
Rao M, Li G, Zhang X et al (2016) Reductive roasting of nickel laterite ore with sodium sulphate for Fe-Ni production. Part II: Phase transformation and grain growth. Sep Sci Technol 51:1727–1735. https://doi.org/10.1080/01496395.2016.1166134
Zhu DQ, Pan J, Li QH, Zheng GL, Li ZY (2011) A process for producing high nickel concentrate from low-grade nickel laterite. China patent, CN 102242252 (in Chinese)
Barberá JJ, Metzger A, Wolf M (2000) Sulfites, thiosulfates, and dithionites. In: Ullmann’s encyclopedia of industrial chemistry, pp 695–704. https://doi.org/10.1002/14356007.a25_477
Abdul F, Pintowantoro S, Yuwandono RB (2018) Analysis of holding time variations to Ni and Fe content and morphology in nickel laterite limonitic reduction process by using coal-dolomite bed. In: AIP Conference Proceedings. American Institute of Physics Inc., p 020033
Abdul F, Pintowantoro S, Kawigraha A, Nursidiq A (2018) Effects of reduction temperature to Ni and Fe content and the morphology of agglomerate of reduced laterite limonitic nickel ore by coal-bed method. In: AIP Conference Proceedings. American Institute of Physics Inc., p 020034
Elliott R, Rodrigues F, Pickles CA, Peacey J (2015) A two-stage thermal upgrading process for nickeliferous limonitic laterite ores. Can Metall Q 54:395–405. https://doi.org/10.1179/1879139515Y.0000000009
Elliott R, Pickles CA, Forster J (2016) Thermodynamics of the reduction roasting of nickeliferous laterite ores. J Miner Mater Charact Eng 04:320–346. https://doi.org/10.4236/jmmce.2016.46028
Diaz CM, Vahed A, Shi D, Doyle CD, Warner AE, Macvicar DJ (1993) Low temperature thermal upgrading of lateritic ores. Australia Patent No. AU2985092A
Zhu D, Pan L, Guo Z et al (2019) Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv Powder Technol 30:451–460. https://doi.org/10.1016/j.apt.2018.11.024
Abdul F, Pintowantoro S, Hidayatullah AB (2019) Analysis of cylindrical briquette dimension on total iron content and the degree of metallization in direct reduction process of iron ore and iron sand mixture. In: Noerochim L (ed) Materials Science Forum, vol 964. Trans Tech Publications Ltd, pp 19–25. https://doi.org/10.4028/www.scientific.net/msf.964.19
Valix M, Cheung WH (2002) Effect of sulfur on the mineral phases of laterite ores at high temperature reduction. Miner Eng 15:523–530. https://doi.org/10.1016/S0892-6875(02)00069-9
Selivanov EN, Sergeeva SV (2019) Prospects for the ferronickel production development from the urals oxidized nickel ores. KnE Mater Sci 5:77. https://doi.org/10.18502/kms.v5i1.3954
Vignes A (2013) Extractive metallurgy 2. Wiley, Hoboken
Chen J, Jak E, Hayes PC (2020) Investigation of the reduction roasting of saprolite ores in the Caron process: effect of sulphur addition. Miner Process Extr Metall Trans Inst Min Metall. https://doi.org/10.1080/25726641.2020.1729020
El-Geassy AHA (2017) Rate controlling step in the reduction of iron oxides; kinetics and mechanism of wüstite-iron step in H2, CO and H2/CO gas mixtures. In: Kin-Tak Lau A (ed) IOP conference series: materials science and engineering, vol 229. Institute of Physics Publishing, p 012002. https://doi.org/10.1088/1757-899X/229/1/012002
Li B, Wang H, Wei Y (2011) The reduction of nickel from low-grade nickel laterite ore using a solid-state deoxidisation method. Miner Eng 24:1556–1562. https://doi.org/10.1016/j.mineng.2011.08.006
Yang S, Du W, Shi P et al (2016) Mechanistic and kinetic analysis of Na2SO4-modified laterite decomposition by thermogravimetry coupled with mass spectrometry. PLoS One 11:e0157369. https://doi.org/10.1371/journal.pone.0157369
Acknowledgements
The authors express sincere gratitude to the Ministry of Research and Technology/National Agency for Research and Innovation Republic of Indonesia for financial funding of this research through Penelitian Tesis Magister Scheme with Contract Number: 3/AMD/E1/KP.PTNBH/2020 and 1408/PKS/ITS/2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Hongmin Zhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pintowantoro, S., Widyartha, A.B., Setiyorini, Y. et al. Sodium Thiosulfate and Natural Sulfur: Novel Potential Additives for Selective Reduction of Limonitic Laterite Ore. J. Sustain. Metall. 7, 481–494 (2021). https://doi.org/10.1007/s40831-021-00352-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00352-4