Abstract
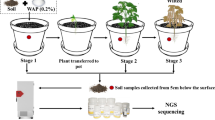
Asparagus (Asparagus officinalis L) is an economically important crop, rich in nutrients, and is also conducive to solving ecological and environmental problems. Plants may acquire benefits from root-associated endophytic bacteria. However, the composition of the endophytic bacterial community associated with the roots of asparagus is poorly elucidated. In this study, the nine root samples of asparagus from three different varieties including Asparagus officinalis var. Grande (GLD), A. officinalis var. Jinglvlu3 (JL3) and A. officinalis var. Jingzilu2 (JZL) were investigated by high-throughput sequencing technology of the 16S rDNA V5-V7 hypervariable region of endophytic bacteria. A total of 16 phyla, 29 classes, 90 orders, 171 families, and 312 genera were identified. Endophytic bacteria diversity and bacteria structure was different among the three varieties and was influenced by rhizosphere soil properties and varieties. In the GLD variety, the main phyla were Proteobacteria, Actinobacteria, and Firmicutes. The main phylum in JL3 and JZL varieties was Proteobacteria. The observations showed that GLD had the highest diversity of endophytes as indicated by the Shannon index (GLD > JZL > JL3). The order of the endophytes richness was GLD > JL3 > JZL. The PCA and PCoA analysis revealed the microbial communities were different between three different asparagus varieties, and the microbial composition of GLD and JZL was more similar. This report provides an important reference for the study of endophytic microorganisms of asparagus.









Similar content being viewed by others
References
Peng L, Yu Y, Zhou K (2015) Analysis of the Industrial layout optimization of asparagus in China. Chin J Agric Resour Reg 36:123–127
Guo Q, Wang N, Liu H, Li Z, Wang C (2019) The bioactive compounds and biological functions of Asparagus officinalis L.——a review. J Funct Foods 65:103727. https://doi.org/10.1016/j.jff.2019.103727
Zhang J, Zhang F, Li D, Liu Y, Liu B, Meng X (2020) Characterization of metabolite profiles of white and green spears of Asparagus officinalis L. from Caoxian, East China. Food Res Int 128:108869. https://doi.org/10.1016/j.foodres.2019.108869
Montero GR (2000) Green asparagus in the meadows of Granada. Agricultura 69:110–115
Huang G et al (2019) Effect of a fungus, Hypoxylon spp. on endophytes in the roots of Asparagus. FEMS Microbiol Lett 16:16. https://doi.org/10.1093/femsle/fnz207
Rather RA, Srinivasan V, Anwar M (2018) Seasonal deviation effects foliar endophyte assemblage and diversity in Asparagus racemosus and Hemidesmus indicus. BMC Ecol. https://doi.org/10.1186/s12898-018-0211-y
Yergeau E, Vujanovic V, St-Arnaud M (2006) Changes in communities of fusarium and arbuscular mycorrhizal fungi as related to different asparagus cultural factors. Microbial Ecol 52:104–113. https://doi.org/10.1007/s00248-006-9047-7
Cole GT, White JF (1985) Fungal endophytes of forage grasses. Indian Acad Sci Sect A Part 3 Math Sci 94:129–135. https://doi.org/10.1007/BF03053132
Firdous J, Lathif NA, Mona R, Muhamad N (2019) Endophytic bacteria and their potential application in agriculture: a review. Indian J Agric Res 53:1–7. https://doi.org/10.18805/IJARe.A-366
Thomas P, Upreti R (2016) Evaluation of tomato seedling root-associated bacterial endophytes towards organic seedling production. Org Agric 6:89–98. https://doi.org/10.1007/s13165-015-0111-9
Thomas P, Swarna GK, Roy PK, Patil P (2008) Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv. Grand Naine. Plant Cell Tissue Organ Cult 93:55–63. https://doi.org/10.1007/s11240-008-9341-9
Mashiane AR, Adeleke R, Bezuidenhout CC, Chirima GJ (2018) Community composition and functions of endophytic bacteria of Bt maize. South Afr J Sci 1:2. https://doi.org/10.17159/sajs.2018/20170018
Compant S, Mitter B, Collimull JG, Gangl H, Sessitsch A (2011) Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microbial Ecol 62:188–197. https://doi.org/10.1007/s00248-011-9883-y
Liu F, Hewezi T, Lebeis SL, Pantalone VR, Grewal PS, Staton M (2019) Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol 19:201–201. https://doi.org/10.1186/s12866-019-1572-x
Huang Y (2019) Illumina-based analysis of endophytic bacterial diversity of four allium species. Sci Rep 9:15271. https://doi.org/10.1038/s41598-019-51707-7
Ou T, Xu W, Wang F, Strobel GA, Zhou Z, Xiang Z, Xie J (2019) A microbiome study reveals seasonal variation in endophytic bacteria among different mulberry cultivars. Comput Struct Biotechnol J. https://doi.org/10.1016/j.csbj.2019.07.018
Lopes KB, Carpentieripipolo V, Oro TH, Pagliosa ES, Degrassi G (2016) Culturable endophytic bacterial communities associated with field-grown soybean. J Appl Microbiol 120:740–755. https://doi.org/10.1111/jam.13046
Zhang Q, Acuna JJ, Inostroza NG, Mora MD, Radic S, Sadowsky MJ, Jorquera MA (2019) Endophytic bacterial communities associated with roots and leaves of plants growing in Chilean extreme environments. Sci Rep 9:4950. https://doi.org/10.1038/s41598-019-41160-x
Izumi H, Anderson IC, Killham KK, Moore ER (2008) Diversity of predominant endophytic bacteria in European deciduous and coniferous trees. Can J Microbiol 54:173–179. https://doi.org/10.1139/W07-134
Riley D, Barber SA (1969) Bicarbonate accumulation and pH changes at the soybean (Glycine max (L.) Merr.) root-soil interface. Soil Sci Soc Am J 33:905–908. https://doi.org/10.2136/sssaj1969.03615995003300060031x
Elmagzob H, Ibrahim MM, Zhang GF (2019) Seasonal diversity of endophytic bacteria associated with cinnamomum camphora (l.) presl. Diversity. https://doi.org/10.3390/d11070112
Bulgarelli D, Garridooter R, Munch PC, Weiman A, Droge J, Pan Y, Schulzelefert P (2015) Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403. https://doi.org/10.1016/j.chom.2015.01.011
Liu Y, Wang R, Li Y, Cao Y, Chen C, Qiu C et al (2017) High-throughput sequencing-based analysis of the composition and diversity of endophytic bacterial community in seeds of “Beijing” hybrid maize planted in china. Plant Growth Regul 81:1–8. https://doi.org/10.1007/s10725-016-0208-5
Jiao R, Munir S, He P, Yang H, He Y (2019) Biocontrol potential of the endophytic bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biol Control 143:104160. https://doi.org/10.1016/j.biocontrol.2019.104160
Kushwaha P, Kashyap PL, Bhardwaj AK, Kuppusamy P, Srivastava AK, Tiwari RK (2020) Bacterial endophyte mediated plant tolerance to salinity: growth responses and mechanisms of action. World J Microbiol Biotechnol 36:1–16. https://doi.org/10.1007/s11274-020-2804-9
Mukasheva T, Berzhanova R, Ignatova LV, Omirbekova A, Brazhnikova YV, Sydykbekova RS, Shigaeva M (2016) Bacterial endophytes of Trans-Ili Alatau region’s plants as promising components of a microbial preparation for agricultural use. Acta Biochim Polonica 63:321–328. https://doi.org/10.18388/abp.2015_1157
Ren Z et al (2018) High-throughput sequencing analysis of endophytic bacteria diversity in fruits of white and red pitayas from three different origins. Pol J Microbiol 67:27. https://doi.org/10.5604/01.3001.0011.6139
Wang SS et al (2019) Diversity of culture-independent bacteria and antimicrobial activity of culturable endophytic bacteria isolated from different Dendrobium stems. Sci Rep. https://doi.org/10.1038/s41598-019-46863-9
Rout ME, Chrzanowski TH (2009) The invasive sorghum halepense harbors endophytic N2-fixing bacteria and alters soil biogeochemistry. Plant Soil 315:163–172. https://doi.org/10.1007/s11104-008-9740-z
Cocking EC (2003) Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 252:169–175. https://doi.org/10.1023/A:1024106605806
Liu S, Guo H, Xu J, Song Z et al (2018) Arbuscular mycorrhizal fungi differ in affecting the flowering of a host plant under two soil phosphorus conditions. J Plant Ecol. https://doi.org/10.1093/jpe/rtx038
Qaisrani MM, Zaheer A, Mirza MS, Naqqash T, Qaisrani TB, Hanif MK, Rasool M (2019) A comparative study of bacterial diversity based on culturable and culture-independent techniques in the rhizosphere of maize (Zea mays L.). Saudi J Biol Sci 26:1344–1351. https://doi.org/10.1016/j.sjbs.2019.03.010
Zhao S, Zhou N, Zhao Z-Y et al (2016) High-throughput sequencing analysis of the endophytic bacterial diversity and dynamics in roots of the halophyte salicornia europaea. Curr Microbiol. https://doi.org/10.1007/s00284-016-0990-3
Community Found in the Soil Cover on the Dried Seabed of the Aral Sea. J Geosci Environ Prot 07:1–23. https://doi.org/10.4236/gep.2019.78001
Peng A, Liu J, Ling W, Chen Z, Gao Y (2015) Diversity and distribution of 16S rRNA and phenol monooxygenase genes in the rhizosphere and endophytic bacteria isolated from PAH-contaminated sites. Sci Rep 5:12173–12173. https://doi.org/10.1038/srep12173
Bashir S, Iqbal A, Hasnain S (2020) Comparative analysis of endophytic bacterial diversity between two varieties of sunflower helianthus annuus with their PGP evaluation. Saudi J Biol Sci 27:720–726. https://doi.org/10.1016/j.sjbs.2019.12.010
Anghinoni FB, Braccini AD, Scapim CA, Anghinoni G, Ferri GC, Suzukawa AK, Tonin TA (2017) Pre-inoc.ulation with Bradyrhizobium spp. in industrially treated soybean seeds. Agric Sci 08:582–590. https://doi.org/10.4236/as.2017.87044
Parker MA, Jankowiak JG, Landrigan GK (2015) Diversifying selection by desmodiinae legume species on Bradyrhizobium symbionts. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiv075
Schippers A, Schumann P, Sproer C (2005) Nocardioides oleivorans sp. Nov., a novel crude oil-degrading bacterium. Int J Syst Evol Microbiol 55:1501–1504. https://doi.org/10.1099/ijs.0.63500-0
Kubota M, Kawahara K, Sekiya K, Uchida T, Hattori Y, Futamata H, Hiraishi A (2005) Nocardioides aromaticivorans sp. Nov., a dibenzofuran-degrading bacterium isolated from dioxin-polluted environments. Syst Appl Microbiol 28:165–174. https://doi.org/10.1016/j.syapm.2004.10.002
Eckelmann D, Spiteller M, Kusari S (2018) Spatial-temporal profiling of prodiginines and serratamolides produced by endophytic Serratia marcescens harbored in Maytenus serrata. Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-23538-5
Amogou O, Dagbenonbakin G, Agbodjato NA, Noumavo PA, Salako KV, Adoko MY, Babamoussa L (2019) Applying rhizobacteria on maize cultivation in northern benin: effect on growth and yield. Agric Sci 10:763–782. https://doi.org/10.4236/as.2019.106059
Passari AK, Mishra VK, Singh G, Singh P, Kumar B, Gupta VK, Singh B (2017) Insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Sci Rep 7:11809–11809. https://doi.org/10.1038/s41598-017-12235-4
Handley LW, Pharr DM, Mcfeeters RF (1983) Carbohydrate changes during maturation of cucumber fruit: implications for sugar metabolism and transport. Plant Physiol 72:498–502. https://doi.org/10.1104/pp.72.2.498
You Y, Park JM, Park J, Kim J (2016) Endophyte distribution and comparative analysis of diversity in wetlands showing contrasting geomorphic conditions. Symbiosis 69:21–36. https://doi.org/10.1007/s13199-015-0363-x
Acknowledgements
The present work was supported by National Key Research & Development (R&D) plan (2017YFD0200603), the Construction and Comprehensive Utilization of Germplasm Bank of Biocontrol Germplasm Resources (KJCX20200203), National Natural Science Foundation of China (NSFC 31460031). We thank Majorbio Cloud Platform for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, Z., Cai, S., Liu, J. et al. Root-Associated Endophytic Bacterial Community Composition of Asparagus officinalis of Three Different Varieties. Indian J Microbiol 61, 160–169 (2021). https://doi.org/10.1007/s12088-021-00926-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-021-00926-6