Abstract
Besides the mode of inheritance, the knowledge of the chromosome location and allelic relationships are the essentials towards a successful deployment and stacking of divergent disease resistance genes for a given pathogen in breeding programs. Powdery mildew of oats, to which 11 major resistance genes in the host Avena sativa L. have been characterized so far, is a prevalent fungal disease of the crop in Northwestern Europe. In the present study, the resistance gene Pm3 was mapped by linkage analysis relative to molecular markers from oat consensus linkage group Mrg18 which was recently determined to represent oat chromosome 1A. Pm3 was located at 67.7–72.6 cM on Mrg18 of the oat consensus map, a position at which also stem and crown rust resistance genes Pg13 and Pc91 and a large cluster of resistance gene analogs have been previously mapped. The closely linked marker GMI_ES03_c2277_336 was found to be useful for the prediction of Pm3 in gene postulation studies. Although the major effect of the widespread gene got lost over time, the known genome location with associated markers will assist revealing in future genetic studies whether there is a possible residual effect of the gene contributing to adult plant resistance.
Similar content being viewed by others
Introduction
Cultivated oat (Avena sativa L.) to which approved health claims have been granted (Storsley et al. 2014) is, though of its high value for human nutrition, low in acreage compared to other cereals like rice, wheat, and maize. The crop is an allohexaploid species (2n = 6x = 42, AACCDD sub-genomes) with a large genome (12.5 Gbp; Yan et al. 2016) and made up of genotypes that contain variable chromosome rearrangements (Singh and Kolb 1991; Chaffin et al. 2016).
Breeding for disease resistance needs to be addressed in every crop breeding program as pathogen attacks can significantly reduce crop yields and grain quality in susceptible cultivars. Powdery mildew of oats, caused by the biotrophic fungus Blumeria graminis, is a major disease in the humid temperate climates of Northwestern Europe (Schwarzbach and Smith 1988; Roderick et al. 2000). Previous studies have shown that losses in grain yield caused by the disease could be attributed to reductions in numbers of fertile panicles and thousand grain weights, while quality parameters such as percentage protein contents and specific weights were negatively correlated with levels of powdery mildew (Roderick and Jones 1988).
Eleven major genes for resistance to powdery mildew in cultivated oats have been catalogued so far (Pm1-Pm11; Hsam et al. 2014; Herrmann and Mohler 2018; Ociepa et al. 2020), but many more sources, as yet uncharacterized, for resistance to powdery mildew do exist in oat landraces and wild relatives of different ploidy levels (Herrmann and Roderick 1996; Okoń et al. 2014, 2016, 2018; Okoń and Kowalczyk 2020). Although the access to genes from lower ploidy levels for enhancing cultivated oat germplasm is challenging (e.g., Aung et al. 1977, 2010; Thomas et al. 1980), agriculture will finally benefit from this work.
The advent of a robust genotyping-by-sequencing approach (Elshire et al. 2011; Huang et al. 2014), i.e., an all-in-one approach combining single nucleotide polymorphism (SNP) discovery and SNP scoring and analyzing pooled samples in a highly multiplexed fashion, allowed establishing the first true road map of the complex oat genome, with 9 of 21 consensus linkage groups unequivocally assigned to physical chromosomes (Chaffin et al. 2016). This map was then substantially extended in the work of Bekele et al. (2018). Recently, a publicly available hexaploid oat genome sequence of oat variety OT3098 was released (https://oatnews.org/).
Of the 11 documented powdery mildew resistance genes, Pm9, Pm10, and Pm11 were allocated to the oat consensus map by GBS marker sequence information (Herrmann and Mohler 2018; Ociepa et al. 2020). It is expected that further consensus map-based genetic mapping studies, e.g., crown rust resistance gene Pc39 was allocated to Mrg11 (Sowa and Paczos-Gręda 2020; Zhao et al. 2020a), Pc53 to Mrg08 (Admassu-Yimer et al. 2018), and Pc98 to Mrg20 (Zhao et al. 2020b), will facilitate determining how resistance genes are distributed across the oat genome. Furthermore, the integrative analysis of historical data from both cytogenetic and genetic mapping studies can further increase this knowledge.
The widespread, dominant resistance gene Pm3, derived from the wild oat A. sterilis L. var. ludoviciana, was assigned to chromosome 17A by monosomic analysis (Hsam and Zeller 1998; Hsam et al. 2014). The gene was mapped relative to restriction fragment length polymorphism (RFLP) markers cMWG706 and cMWG733 from homoeologous group-1 chromosomes of the Triticeae (Mohler et al. 2012). In the present study, we used these historical data for the targeted re-mapping of Pm3. Furthermore, we explored the prediction potential of SNP markers with linkage to Pm3.
Materials and methods
Plant materials and DNA isolation
The Pm3 mapping population Kanota × Rollo comprised 79 F2:3 lines and was reported in Mohler et al. (2012). A set of 104 oat cultivars/lines (Table S1) was used to assess the genotype frequency and the predictive ability of SNP markers linked to Pm3. For the diversity panel, genomic DNA was extracted from lyophilized primary wheat leaves as described by Plaschke et al. (1995). For 53 oats of the collection, the Pm3 and other Pm phenotypes were known from previous studies (Hsam et al. 1997, 1998, 2014; Yu and Herrmann 2006; Herrmann and Mohler 2018). The pedigrees of the oat lines carrying Pm3 (Table S2) were accessed from the POOL database (Tinker and Deyl 2005; https://triticeaetoolbox.org/POOL/).
Phenotypic data
The phenotypic data for Kanota × Rollo originated from Mohler et al. (2012) and are based on seedling inoculation tests that used 12 to 16 plants for each F2:3 line. The isolate HGB2/1, known to carry avirulence for Pm3 from monosomic analysis of powdery mildew resistance in Rollo (Hsam et al. 2014), was spread in a settling tower on leaf segments at densities of 400–500 spores/cm2. The leaf segments were cultured in plastic dishes on 6 g/l agar and 35 mg/l benzimidazole. The conditions of incubation were under continuous lighting at 10 μE/m2s in a growth chamber at 17 °C and at 70% relative humidity. Ten days after inoculation, two classes of host reactions relative to the susceptible control cultivar Fuchs were distinguished: resistant (0–20%) and susceptible (> 50% infection); no intermediate (30–50%) infections were observed.
BLASTn analysis
Similarity to oat DNA sequences was searched for barley cDNA RFLP markers cMWG704 and cMWG733 by using their DNA sequences as queries against genetically mapped sequences and the hexaploid oat genome assembly lodged in the T3/oat database using default settings (https://triticeaetoolbox.org/oat/). The RFLP marker sequences were obtained from the GrainGenes database (https://wheat.pw.usda.gov). Matched sequences as well as all other oat marker sequences from the target linkage group Mrg18 used for genetic mapping were subsequently blasted against the wheat reference genome sequence (RefSeq_v1.0; International Wheat Genome Sequencing Consortium, 2018) in the Ensembl Plants database (http://plants.ensembl.org/index.html). The function of the detected high confidence protein-coding genes was retrieved from the T3/wheat database (https://triticeaetoolbox.org/wheat/) (Tables S3 and S4).
Genetic mapping
A total of 32 framework markers consisting of 6 K array SNP markers (GMI) and GBS markers (avgbs) and distributed along the linkage group Mrg18 of the oat consensus map (Chaffin et al. 2016) were used for SNP development and data collection (Table S3). The SNP assays were designed by Fluidigm Corporation (South San Francisco, USA). SNP marker genotypes were recorded on an EP1 genotyping platform using 192.24 Dynamic Array integrated fluidic circuits. All SNP genotyping analysis protocols can be found in the user guide published by the manufacturer (https://www.fluidigm.com). Genotyping with polymorphic SNP markers was done in double. The SNP data of the mapping population Kanota × Rollo were merged with previously established genotypic data, i.e., RFLP and amplified fragment length polymorphism (AFLP) markers, and the binary Pm3 phenotype (Mohler et al. 2012). To avoid complications in positioning tightly linked dominant markers from opposite linkage phases as accurately as possible (Mester et al. 2003), two separate but related linkage maps, both share the co-dominant markers, were computed with JoinMap® software version 5.0 (Kyazma BV, Wageningen, The Netherlands). The “maternal” map included dominant markers that were scored as heterozygous in the female parent (Kanota) and homozygous in the male parent (Rollo), while “paternal” markers were heterozygous in Rollo and homozygous in Kanota. Linkage of loci was claimed at a logarithm of the odds ratio (LOD) score ≥ 3.0, with a maximum recombination fraction of 0.4. Regression mapping was performed using the Haldane mapping function. Genetic linkage maps were drawn with Mapchart 2.1 software (Voorrips 2002). Chi-squared tests for goodness of fit were used to test for deviation of observed data from theoretically expected segregation ratios. Chi-squared values were corrected for continuity (http://vassarstats.net/csfit.html).
Results
Assignment of Pm3 to linkage group Mrg18 of the oat consensus map
BLASTn search for the cDNA sequence of barley RFLP marker cMWG706, which is closely linked to oat powdery mildew resistance gene Pm3, in the T3/oat database produced significant alignments to GBS markers avgbs_249172 (bit score 80.6), avgbs2_5635 (bit score 78.8), avgbs2_165855 (bit score 73.4), and avgbs_670066 (bit score 66.2) of which avgbs_249172 and avgbs2_5635 represent the same sequence stretch (Fig. S1). The markers avgbs2_5635 and avgbs2_165855 were known to map at 69.9 cM and 20.2 cM, respectively, of linkage group Mrg18 of the oat consensus map (2016ExpandedConsensus_Mrg18). All five sequences identified the same high confidence protein-coding gene, a phosphoethanolamine methyltransferase triplicated on the long arm of homoeologous group-1 chromosomes, in the wheat reference genome sequence of Chinese Spring (Table S4). No significant hit to genetically mapped oat markers was obtained for cMWG733; however it produced, as cMWG706, significant alignments with oat chromosomes 1A and 1D and targeted a high confidence protein-coding gene of unknown function located on each of the three homoeologous group-1 chromosomes in the wheat reference genome sequence (Table S4).
Genetic mapping of Pm3 relative to SNP markers from Mrg18
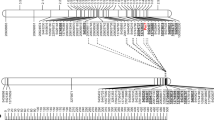
Of the 32 framework markers mainly chosen from the 56.0–76.7 cM region of linkage group Mrg18 of the oat consensus map, 13 showed polymorphism between the parental lines of the mapping population, 14 were monomorphic and 5 were failures (Table S3). Four SNP markers showed co-dominant inheritance, while all nine dominant markers were heterozygous for the paternal parent Rollo. The maternal genetic map had a map length of 13.1 cM and comprised seven markers including co-dominant RFLP marker cMWG706 and two dominant markers, one RFLP and AFLP each, from Mohler et al. (2012) (Fig. 1a). The resistance gene was bracketed by the RFLP marker loci cmwg706_DraI and cmwg733_HindIII at map distances of 1.1 cM and 4.0 cM, respectively. The closest SNP marker locus was GMI_ES03_c2277_336 and mapped 3.8 cM proximal to Pm3. The paternal genetic map contained 17 markers and spanned a map distance of 31.7 cM (Fig. 1b). Two AFLP markers, E37M53-398 and E42M56-149, completed the data set. Eight markers were located within a 3.2 cM-interval around Pm3: 4 SNPs, 3 AFLPs, and RFLP cMWG706. No linkage in both maps was found for GMI_ES02_lrc12474_560 and GMI_ES03_c609_767 located at 36.2 cM and 56.0 cM, respectively, on consensus linkage group Mrg18. Six and 13 molecular markers had distorted segregation ratios in the maternal and the paternal map, respectively (Table S5). The marker with the greatest degree of segregation distortion in the Kanota × Rollo linkage group was GMI_ES03_c2277_336. The Pm3 resistance locus and most of the SNP markers showed a deficiency of Kanota—the female parent—homozygotes.
Maternal a and b paternal genetic maps for the linkage group representing Mrg18 of the oat consensus map in mapping population Kanota × Rollo. Co-dominant markers are common to both maps. Absolute map positions in cM and marker names are shown on the left and right sides, respectively, of each genetic map
Evaluation of the predictive ability of SNP markers for Pm3
Table S1 shows the genotypes and the genotype frequencies of the diversity panel for the 11 mapped SNP markers where the markers were ordered according to their position on the consensus linkage group Mrg18. Altogether, seven markers showed genotype frequencies ≤ 5% in the set of the 104 oat samples. For the remaining four markers, the minor genotype frequency ranged between 0.12 and 0.39.
For 53 oats of the genotype collection, the status of major powdery mildew resistance genes was known: 15 oats carried Pm3 singly or in combination, 19 possessed other Pm genes, one was postulated to have an unknown Pm gene, and 18 reacted susceptible. Looking at these 53 oats, GMI_ES03_c2277_336 showed a distribution of genotype A:A similar to Pm3. Except for the oat cultivars Hinoat and Pendrwm, which were postulated to carry Pm gene combinations, the prediction of the presence or absence of Pm3 in the validation set was accurate (Table 1). The Swedish oat cultivar Galopp carrying Pm3 was heterozygous for GMI_ES03_c2277_336. When extending the prediction to the full panel, oat cultivars AC Marie, Curley and Keely were suggested to carry Pm3 (Table S1). Two other markers, GMI_ES15_c1671_378 and GMI_ES15_c4142_273, were also evaluated for their potential to predict Pm3 in the sub-panel. However, prediction was limited for both markers. There were seven (1 false-positive and 6 false-negatives) and eight misclassifications (2 false-positives and 6 false-negatives) for GMI_ES15_c1671_378 and GMI_ES15_c4142_273, respectively (Table 1). For both markers, false-negative classifications were common to Mostyn and its derivatives Avalanche, Johanna and Manoire. The remaining 2 false-negative predictions were for Hinoat and Pendrwm which already were wrongly determined by GMI_ES03_c2277_336. Cultivar Galopp and Pm10-carrier AVE2925 showed a heterozygous allele configuration for both markers. The prediction for Cc 4146 possessing Pm1 by GMI_ES15_c4142_273 was rated as false-positive.
Discussion
The present study reported the genetic mapping of the oat powdery mildew resistance gene Pm3 relative to SNP markers derived from linkage group Mrg18 (recently determined to represent oat chromosome 1A) of the oat consensus map, and thus helped to gather information about the distribution of disease resistance genes across the oat genome. The initial identification of the target consensus linkage group was achieved by BLASTn similarity search for barley cDNA RFLP maker cMWG706, which was previously mapped in the vicinity of Pm3, in oat DNA sequences for which genetic map locations were also lodged in a database. The precise location of the matched oat marker allowed targeted marker enrichment for the genome region containing Pm3. The RFLP makers cMWG706 and cMWG733 (Table S4) and other 13 SNP markers chosen for the genetic mapping study (Table S3) identified high confidence protein-coding genes in the wheat reference genome sequence which all were located on each of the three homoeologous group-1 chromosomes further confirming the relationship of these chromosomes from oats and wheat (Jellen et al. 1995).
The powdery mildew resistance gene was located at 67.7–72.6 cM on Mrg18 of the oat consensus map, a position similar to the widely deployed oat stem rust resistance gene Pg13 and crown rust resistance gene Pc91 recently described in Kebede et al. (2020) indicating a possible clustering of disease resistance genes in this region of the oat genome. The likely occurrence of disease resistance gene clusters in the oat genome was recently predicted by annotating resistance gene analogs in the genomes of the diploid A. atlantica (AsAs) and A. eriantha (CpCp) species (Maughan et al. 2019). A cluster of resistance gene analogs on Mrg18 coincided with SNP marker GMI_ES03_c2277_336 which was found to be closely linked to Pm3 in this study. In the study of Oliver et al. (2013), Mrg18 was associated with physical chromosomes assumed to carry the reciprocal translocation 7C‐17A which is common to cultivated oats (Jellen and Beard 2000). In addition, the results presented by Kebede et al. (2020) indicated that Pg13 and Pc91 are near the 7C‐17A translocation breakpoint. It is not clear whether this is also true for Pm3. However, a significant portion of the markers located on Mrg18 in the Kanota × Rollo population showed distorted segregation similar to the markers that were mapped to Mrg18 in the AC Morgan × CDC Morrison Pg13 mapping population for which variation for the 7C‐17A translocation was assumed (Kebede et al. 2020). Distorted segregation is often observed in crosses involving parental lines with and without the 7C‐17A translocation (Wight et al. 2003). As our maternal line A. byzantina cv. Kanota does not carry the translocation (Jellen and Beard 2000), the skewed segregation of markers in the population could be due to the presence of this widespread chromosome mutation in the paternal line Rollo. In previous monosomic analyses involving the Pm3-carriers Mostyn (Hsam and Zeller 1998) and Rollo (Hsam et al. 2014), Pm3 was located on chromosome 17A corresponding to the missing chromosome in the Kanota monosomic line K11 which was involved in the critical crosses. Thus, it can be concluded that the linkage group in Kanota × Rollo corresponding to consensus linkage group Mrg18 represents chromatin from chromosome 17A. This conclusion is further supported by the observation that the part of Mrg18 targeted by GMI_ES03_c2277_336 corresponds to the distal part of chromosome AA2 of the diploid A-genome accession A. atlantica (Maughan et al. 2019).
Except for the cultivars Pendrwm and Hinoat, which were postulated to carry gene combinations, SNP marker GMI_ES03_c2277_336 showed a high prediction in the validation panel of 53 oats. It appears that there must be no doubt about previous gene postulations as the reaction patterns of Pendrwm and Hinoat fully corresponded to a combination of the reaction pattern of single-gene lines for Pm1 and Pm3 and Pm3 and U from cultivar Extraklock, respectively (Hsam et al. 1997, 1998). However, the haplotypes of Pendrwm and Hinoat across the eleven mapped markers were similar to many other oats from the diversity panel (Table S1). In contrast to line 9065 Cn 18/53, which was the primary resistance source for the distribution of Pm3 in oat cultivars and for which the marker prediction was true, line CD 3820, a diploid oat from the species A. strigosa recognized to carry Pm3 (Hsam et al. 1997) and involved in the development of cultivar Hinoat, was not available for genotyping. The cultivar Mostyn, for which 9065 Cn 18/53 via line 05,443 was used as Pm3 source, and its derivatives Avalanche, Johanna and Manoire were correctly determined by SNP marker GMI_ES03_c2277_336. This was also observed for Pm3 resistance derived from sources of unknown origin in cultivars Pewi, Fuwi, Lowi, Rollo, Nordstern and breeding line NIC-91–7026 (Hsam et al. 1997). Barra was derived from a cross Selma//Palu/Saxo. The cultivar Palu in turn was developed from the cross Seger/von Lochow’s Gelbhafer//Minor. As cultivars Minor, Selma and Saxo were susceptible (Hsam et al. 1998), Seger, which is a selection from the Swedish cultivar Milton, or von Lochow’s Gelbhafer could have contributed the Pm3 resistance in cultivar Barra. For cultivar Galopp, which was heterozygous for GMI_ES03_c2277_336, the origin of Pm3 is even less clear (Hsam et al. 1998).
Although Pm3 became ineffective over time (Okoń 2015; Okoń and Tociepa 2017) possibly due to its frequent use as sole resistance component in cultivars, the usefulness of Pm3 in gene combinations is unknown, especially when there is high virulence for the gene in oat powdery mildew field populations. The knowledge of the genome location of Pm3 and the availability of a highly informative linked marker will help in future QTL mapping studies to recognize whether this defeated gene contributes to adult plant resistance to powdery mildew. The availability of a consensus map along with its reference genome sequence has been found to be highly useful for the purpose of expanding the knowledge of the genetics of powdery mildew resistance in cultivated oat.
Change history
03 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42976-021-00165-x
References
Admassu-Yimer B, Bonman JM, Klos KE (2018) Mapping of crown rust resistance gene Pc53 in oat (Avena sativa). PLoS ONE 13:e0209105. https://doi.org/10.1371/journal.pone.0209105
Aung T, Thomas H, Jones IT (1977) The transfer of the gene for mildew resistance from Avena barbata (4×) into the cultivated oat A. sativa by an induced translocation. Euphytica 26:623–632. https://doi.org/10.1007/BF00021687
Aung T, Zwer P, Park R, Davies P, Sidhu P, Dundas I (2010) Hybrids of Avena sativa with two diploid wild oats (Clav6956) and (Clav7233) resistant to crown rust. Euphytica 174:189–198. https://doi.org/10.1007/s10681-009-0111-5
Bekele WA, Wight CP, Chao SM, Howarth CJ, Tinker NA (2018) Haplotype-based genotyping-by-sequencing in oat genome research. Plant Biotechnol J 16:1452–1463. https://doi.org/10.1111/pbi.12888
Chaffin AS, Huang YF, Smith S, Bekele WA, Babiker E, Gnanesh BN, Foresman BJ, Blanchard SG, Jay JJ, Reid RW, Wight CP, Chao S, Oliver R, Islamovic E, Kolb FL, McCartney C, Mitchell Fetch JW, Beattie AD, Bjørnstad Å, Bonman JM, Langdon T, Howarth CJ, Brouwer CR, Jellen EN, Klos KE, Poland JA, Hseih TF, Brown R, Jackson E, Schlueter JA, Tinker NA (2016) A consensus map in cultivated hexaploid oat reveals conserved grass synteny with substantial subgenome rearrangement. Plant Genome 9:2. https://doi.org/10.3835/plantgenome2015.10.0102
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6:e19379. https://doi.org/10.1371/journal.pone.0019379
Herrmann MH, Mohler V (2018) Locating two novel genes for resistance to powdery mildew from Avena byzantina in the oat genome. Plant Breed 137:832–838. https://doi.org/10.1111/pbr.12655
Herrmann M, Roderick HW (1996) Characterisation of new oat germplasm for resistance to powdery mildew. Euphytica 89:405–410. https://doi.org/10.1007/BF00022300
Hsam SLK, Zeller FJ (1998) Chromosomal location of genes for resistance to powdery mildew in cultivated oat (Avena sativa L.). 1. Gene Eg-3 in the cultivar ‘Mostyn.’ Plant Breed 117:177–178. https://doi.org/10.1111/j.1439-0523.1998.tb01474.x
Hsam SLK, Peters N, Paderina EV, Felsenstein F, Oppitz K, Zeller FJ (1997) Genetic studies of powdery mildew resistance in common oat (Avena sativa L.) I. Cultivars and breeding lines grown in Western Europe and North America. Euphytica 96:421–427. https://doi.org/10.1023/A:1003057505151
Hsam SLK, Paderina EV, Gordei S, Zeller FJ (1998) Genetic studies of powdery mildew resistance in cultivated oat (Avena sativa L.) cultivars and breeding lines grown in Northern and Eastern Europe. Hereditas 129:227–230. https://doi.org/10.1111/j.1601-5223.1998.00227.x
Hsam SLK, Mohler V, Zeller FJ (2014) The genetics of resistance to powdery mildew in cultivated oats (Avena sativa L.): current status of major genes. J Appl Genet 55:155–162. https://doi.org/10.1007/s13353-014-0196-y
Huang YF, Poland JA, Wight CP, Jackson EW, Tinker NA (2014) Using genotyping-by-sequencing (GBS) for genomic discovery in cultivated oat. PLoS ONE 9:e102448. https://doi.org/10.1371/journal.pone.0102448
Jellen EN, Beard J (2000) Geographical distribution of a chromosome 7C and 17 intergenomic translocation in cultivated oat. Crop Sci 400:256–263. https://doi.org/10.2135/cropsci2000.401256x
Jellen EN, Phillips RL, Rooney WL, Rines HW (1995) Molecular genetic identification of Avena chromosomes related to the group 1 chromosomes of the Triticeae. Genome 38:185–189. https://doi.org/10.1139/g95-023
Kebede AZ, Admassu-Yimer B, Bekele WA, Gordon T, Bonman JM, Babiker E, Jin Y, Gale S, Wight CP, Tinker NA, Menzies GJ, Beattie AD, Mitchell Fetch JW, Fetch TG, Esvelt Klos K, McCartney CA (2020) Mapping of the stem rust resistance gene Pg13 in cultivated oat. Theor Appl Genet 133:259–270. https://doi.org/10.1007/s00122-019-03455-5
Maughan PJ, Lee R, Walstead R, Vickerstaff RJ, Fogarty MC, Brouwer CR, Reid RR, Jay JJ, Bekele WA, Jackson EW, Tinker NA, Langdon T, Schlueter JA, Jellen EN (2019) Genomic insights from the first chromosome-scale assemblies of oat (Avena ssp.) diploid species. BMC Biol 17:92. https://doi.org/10.1186/s12915-019-0712-y
Mester DI, Ronin YI, Hu Y, Peng J, Nevo E, Korol AB (2003) Efficient multipoint mapping: making use of dominant repulsion-phase markers. Theor Appl Genet 107:1102–1112. https://doi.org/10.1007/s00122-003-1305-1
Mohler V, Zeller FJ, Hsam SLK (2012) Molecular mapping of powdery mildew resistance gene Eg-3 in cultivated oat (Avena sativa L. cv. ‘Rollo’). J Appl Genet 53:145–148. https://doi.org/10.1007/s13353-011-0077-6
Ociepa T, Okoń S, Nucia A, Leśniowska-Nowak J, Paczos-Grzęda E, Bisaga M (2020) Molecular identification and chromosomal localization of new powdery mildew resistance gene Pm11 in oat. Theor Appl Genet 133:179–185. https://doi.org/10.1007/s00122-019-03449-3
Okoń SM (2015) Effectiveness of resistance genes to powdery mildew in oat. Crop Prot 74:48–50. https://doi.org/10.1016/j.cropro.2015.04.004
Okoń S, Kowalczyk K (2020) Screening oat landraces for resistance to Blumeria graminis f. sp. avenae. J Plant Pathol 10:100. https://doi.org/10.1007/s42161-020-00506-5
Okoń SM, Ociepa T (2017) Virulence structure of the Blumeria graminis DC f. sp. avenae populations occurring in Poland across 2010–2013. Eur J Plant Pathol 149:711–718. https://doi.org/10.1007/s10658-017-1220-y
Okoń SM, Chrząstek M, Kowalczyk K, Koroluk A (2014) Identification of new sources of resistance to powdery mildew in oat. Eur J Plant Pathol 139:9–12. https://doi.org/10.1007/s10658-013-0367-4
Okoń S, Paczos-Gręda E, Ociepa T, Koroluk A, Sowa S, Kowalczyk K, Chrząstek M (2016) Avena sterilis L. genotypes as a potential source of resistance to oat powdery mildew. Plant Dis 100:2145–2151. https://doi.org/10.1094/PDIS-11-15-1365-RE
Okoń SM, Ociepa T, Paczos-Grzęda E, Ladizinsky G (2018) Evaluation of resistance to Blumeria graminis (DC.) f. sp. avenae, in Avena murphyi and A. magna genotypes. Crop Prot 106:177–181. https://doi.org/10.1016/j.cropro.2017.12.025
Oliver RE, Tinker NA, Lazo GR, Chao S, Jellen EN, Carson ML, Rines HW, Obert DE, Lutz JD, Shackelford I, Korol AB, Wight CP, Gardner KM, Hattori J, Beattie AD, Bjørnstad Å, Bonman JM, Jannink J-L, Sorrells ME, Brown-Guedira GL, Mitchell Fetch JW, Harrison SA, Howarth CJ, Ibrahim A, Kolb FL, McMullen MS, Murphy JP, Ohm HW, Rossnagel BG, Yan W, Miclaus KJ, Hiller J, Maughan PJ, Redman Hulse RR, Anderson JM, Islamovic E, Jackson EW (2013) SNP discovery and chromosome anchoring provide the first physically-anchored hexaploid oat map and reveal synteny with model species. PLoS ONE 8:e58068. https://doi.org/10.1371/journal.pone.0058068
Plaschke J, Ganal MW, Röder MS (1995) Detection of genetic diversity in closely related bread wheat using microsatellite markers. Theor Appl Genet 91:1001–1007. https://doi.org/10.1007/BF00223912
Roderick HW, Jones IT (1988) The effects of powdery mildew (Erysiphe graminis f. sp. avenae) on yield, yield components and grain quality of spring oats. Ann Appl Biol 113:455–460. https://doi.org/10.1111/j.1744-7348.1988.tb03323.x
Roderick HW, Jones ERL, Šebesta J (2000) Resistance to oat powdery mildew in Britain and Europe: a review. Ann Appl Biol 136:85–91. https://doi.org/10.1111/j.1744-7348.2000.tb00012.x
Schwarzbach E, Smith IM (1988) Erysiphe graminis DC. In: Smith IM, Dunez J, Lelliot RA, Phillips DH, Archer SA (eds) European handbook of plant diseases. Blackwell, Oxford
Singh RJ, Kolb FL (1991) Chromosomal interchanges in six hexaploid oat genotypes. Crop Sci 31:726–729. https://doi.org/10.2135/cropsci1991.0011183X003100030038x
Sowa S, Paczos-Gręda E (2020) Identification of molecular markers for the Pc39 gene conferring resistance to crown rust in oat. Theor Appl Genet 133:1081–1094. https://doi.org/10.1007/s00122-020-03533-z
Storsley J, Jew S, Ames N (2014) Health claims for oat products: a global perspective. In: Chu Y (ed) Oats nutrition and technology. Wiley
Thomas H, Powell W, Aung T (1980) Interfering with regular meiotic behaviour in Avena sativa as a method of incorporating the gene for mildew resistance from A. barbata. Euphytica 29:635–640. https://doi.org/10.1007/BF00023211
Tinker NA, Deyl JK (2005) A curated internet database of oat pedigrees. Crop Sci 43:2269–2272. https://doi.org/10.2135/cropsci2004.0687
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Wight CP, Tinker NA, Kianian SF, Sorrells ME, O’Donoughue LS, Hoffman DL, Groh S, Scoles GJ, Li CD, Webster FH, Phillips RL, Rines HW, Livingston SM, Armstrong KC, Fedak G, Molnar SJ (2003) A molecular marker map in ‘Kanota’ x ‘Ogle’ hexaploid oat (Avena spp.) enhanced by additional markers and a robust framework. Genome 46:28–47. https://doi.org/10.1139/g02-099
Yan H, Martin SL, Bekele WA, Latta RG, Diederichsen A, Peng Y, Tinker NA (2016) Genome size variation in the genus Avena. Genome 59:209–220. https://doi.org/10.1139/gen-2015-0132
Yu J, Herrmann M (2006) Inheritance and mapping of powdery mildew resistance gene introgressed from Avena macrostachya in cultivated oat. Theor Appl Genet 113:429–437. https://doi.org/10.1007/s00122-006-0308-0
Zhao J, Kebede AZ, Bekele WA, Menzies GJ, Chong J, Mitchell Fetch JW, Tinker NA, Beattie AD, Peng YY, McCartney CA (2020a) Mapping of the oat crown rust resistance gene Pc39 relative to single nucleotide polymorphism markers. Plant Dis. https://doi.org/10.1094/PDIS-09-19-2002-RE
Zhao J, Kebede AZ, Menzies GJ, Paczos-Gręda E, Chong J, Mitchell Fetch JW, Beattie AD, Peng YY, McCartney CA (2020b) Chromosomal location of the crown rust resistance gene Pc98 in cultivated oat (Avena sativa L.). Theor Appl Genet 133:1109–1122. https://doi.org/10.1007/s00122-020-03535-x
Acknowledgements
The author thanks Sabine Schmidt (greenhouse work) and Petra Greim (molecular lab work) for excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
VM conceived the research, performed all data analyses and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Communicated by Á. Mesterházy.
The original version of this article has been revised: Table 1 and Table S1 (in the supplementary material) have been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohler, V. Allocation of the oat powdery mildew resistance gene Pm3 to oat chromosome 1A. CEREAL RESEARCH COMMUNICATIONS 50, 1–8 (2022). https://doi.org/10.1007/s42976-021-00152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-021-00152-2