Abstract
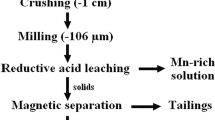
Iron and manganese both are important ferrous metals, and they usually need to be extracted separately for better usage. Ferromanganese ore from the Yunnan Province of China was investigated by subjecting it to reduction roasting, leaching, magnetic separation, hydrometallurgical separation process, X-ray diffraction (XRD), electron probe micro-analysis (EPMA) and scanning electron microscopy/energy-dispersive spectroscopy (SEM-EDS). It is noted that the ferromanganese ore contains valuable components or elements of 21.41 wt% Fe2O3 and 19.88 wt% MnO, which are 14.99% and 15.40% of the total of Fe and Mn, respectively, with a large amount of impurities comprising 33.00 wt% SiO2, 9.81%wt% Al2O3, etc. After roasting, microscopic examination showed the presence of haematite, magnetite, pyrolusite, psilomelane, hollandite, marcasite, pyrite, limonite, braunite and manganite, while XRD analysis showed that the magnetite content increases with the increase in roasting temperature, reflecting the increase in conversion of haematite to magnetite. Based on EPMA and SEM-EDS analysis, it was shown that the haematite and magnetite interface were the main Fe-bearing minerals; however, the dissemination of the two minerals, that is, haematite and magnetite with the coexistence of Fe- and Mn-bearing minerals, will result in difficulty in effective separation of Fe and Mn. The effective roasting temperature and roasting time were 800 °C and 60 min, respectively, to avoid the formation of a composite oxide of MnxFe3-xO4 beyond 800 °C. The Fe recovery via roasting and magnetic separation only was 66.56%, whereas a combination of magnetic separation which ensued after the leaching process showed a recovery of 80.08% and 59.95% of Fe and Mn, respectively.



















Similar content being viewed by others
References
Wills BA, Napier-Munn TJWMPT (2006) An introduction to the practical aspects of ore treatment and mineral recovery p. 267–352
Ponomar VP, Dudchenko NO, Brik AB (2017) Reduction roasting of hematite to magnetite using carbohydrates. Int J Miner Process 164:21–25
Xiao J et al (2017) Comparison between Datangpo-type manganese ores and modern marine ferromanganese oxyhydroxide precipitates based on rare earth elements. Ore Geol Rev 89:290–308
Peng N et al (2018) Recovery of iron and manganese from iron-bearing manganese residues by multi-step roasting and magnetic separation. Miner Eng 126:177–183
Luo L-q, Zhang H-q (2017) Process mineralogy and characteristic associations of iron and phosphorus-based minerals on oolitic hematite. J Cent South Univ 24(9):1959–1967
Song S-x et al (2013) Morphological and mineralogical characterizations of oolitic iron ore in the Exi region, China. Int J Miner Metall Mater 20(2):113–118
Liu B et al (2019) Extraction and separation of manganese and iron from ferruginous manganese ores: A review. Miner Eng 131:286–303
Hansen JS, Tress JE, Nafziger RH (1993) Carbothermic reduction of U.S. ferruginous manganese resources. JOM 45(4):59–63
Swamy YV et al (1998) Enrichment of the manganese to iron ratio of ferruginous low-grade manganese ore using solid reductant. Mining. Metall Explor 15(3):34–37
Barner HE, Mantell CL (1968) Kinetics of hydrogen reduction of manganese dioxide. Ind Eng Chem Process Des Dev 7(2):285–294
El-Geassy AA et al (2000) Behaviour of manganese oxides during magnetising reduction of Baharia iron ore by CO–CO2 gas mixture. Ironmak Steelmak 27(2):117–122
El-Geassy A-HA et al (2007) Reduction kinetics and catastrophic swelling of MnO2−doped Fe2O3 Compacts with CO at 1073–1373 K. ISIJ Int 47(3):377–385
El-Geassy A-HA et al (2008) Influence of SiO2 and/or MnO2 on the Reduction Behaviour and Structure Changes of Fe2O3 Compacts with CO Gas. ISIJ Int 48(10):1359–1367
Anacleto N, Ostrovski O, Ganguly S (2004) Reduction of manganese ores by methane-containing gas. ISIJ Int 44(10):1615–1622
Ostrovski O, Zhang G (2006) Reduction and carburization of metal oxides by methane-containing gas. AICHE J 52(1):300–310
Kononov R, Ostrovski O, Ganguly S (2008) Carbothermal reduction of manganese oxide in different gas atmospheres. Metall Mater Trans B Process Metall Mater Process Sci 39(5):662–668
Gao Y et al (2012) Retraction: Gaseous pre-reduction for the magnetic beneficiation of ferruginous low-grade Mn ore. ISIJ Int 52(5):759–763
Gao Y et al (2012) Upgrading of low-grade manganese ore by selective reduction of iron oxide and magnetic separation. Metall Mater Trans B Process Metall Mater Process Sci 43(6):1465–1475
Liu B et al (2017) Thermodynamic analysis and reduction of MnO2 by methane–hydrogen as mixture. JOM 69(9):1669–1675
Liu B et al (2018) A study on the carbonization and alloying process of MnO2 by methane-hydrogen gas mixture in the presence of Fe2O3. Powder Technol 325:271–279
Liu B et al (2018) New understanding on separation of Mn and Fe from ferruginous manganese ores by the magnetic reduction roasting process. Appl Surf Sci 444:133–144
Abdel Halim KS (2007) Isothermal reduction behavior of Fe2O3/MnO composite materials with solid carbon. Mater Sci Eng A 452-453:15–22
Cheng Z, Zhu G, Zhao Y (2009) Study in reduction-roast leaching manganese from low-grade manganese dioxide ores using cornstalk as reductant. Hydrometallurgy 96(1):176–179
Abdel Halim KS et al (2011) Pre-reduction of manganese ores for ferromanganese industry. Ironmak Steelmak 38(4):279–284
Gao L et al (2019) Upgrading of low-grade manganese ore based on reduction roasting and magnetic separation technique. Sep Sci Technol 54(1):195–206
Mpho M, Samson B, Ayo A (2013) Evaluation of reduction roasting and magnetic separation for upgrading Mn/Fe ratio of fine ferromanganese. Int J Min Sci Technol 23(4):537–541
Chen TT, Dutrizac JE (2004) Mineralogical changes occurring during the fluid-bed roasting of zinc sulfide concentrates. Jom 56(12):46–51
Pagnanelli F et al (2004) Leaching of low-grade manganese ores by using nitric acid and glucose: optimization of the operating conditions. Hydrometallurgy 75(1):157–167
El Hazek MN, Lasheen TA, Helal AS (2006) Reductive leaching of manganese from low grade Sinai ore in HCl using H2O2 as reductant. Hydrometallurgy 84(3):187–191
Nayl AA, Ismail IM, Aly HF (2011) Recovery of pure MnSO4∙H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int J Miner Process 100(3):116–123
Gao Q et al (2013) Preparation and characterization of activated carbon from wool waste and the comparison of muffle furnace and microwave heating methods. Powder Technol 249:234–240
Lee RJ et al (2016) Impact of muffle furnace preparation on the results of crystalline silica analysis. Regul Toxicol Pharmacol 80:164–172
Rai S et al (2019) Recovery of iron from bauxite residue using advanced separation techniques. Miner Eng 134:222–231
Faris N et al (2017) Investigation into coal-based magnetizing roasting of an iron-rich rare earth ore and the associated mineralogical transformations. Miner Eng 114:37–49
Zhou Y et al (2017) Separation and Recovery of Iron and Rare Earth from Bayan Obo Tailings by Magnetizing Roasting and (NH4)(2)SO4 Activation Roasting. Metals 7(6)
Rath SS et al (2018) Characterization and reduction roasting studies of an iron rich manganese ore. 71(4):861–872
Brough CP et al (2013) The process mineralogy of mine wastes. Miner Eng 52:125–135
Lotter NO et al (2011) Modern Process Mineralogy: Two case studies. Miner Eng 24(7):638–650
Moradkhani D et al (2014) Recovery of valuable metals from zinc plant residue through separation between manganese and cobalt with nn reagent. Physicochem Probl Min Process 50(2):735–746
Montes-Sotomayor S, Houot R, Kongolo M (1998) Flotation of silicated gangue iron ores: Mechanism and effect of starch. Miner Eng 11(1):71–76
Jung YH, Baik SJ, Ahn SB (2019) Investigation of Zircaloy-fuel interaction in failed spent PWR fuel using EPMA. J Nucl Mater 517:349–355
Acknowledgments
The authors gratefully acknowledge financial support by the State Natural Science Foundation of China (no. 51874219). The authors would like to extend their sincere thanks to the China University of Geosciences, especially teacher Manrong Jiang for her support during microscopy analysis. Our feelings of gratitude are also addressed to teacher Jin Feng Chen of the School of Resources and Environmental Engineering at Wuhan University of Technology for her kindly support in chemical analyses of different samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niyonzima, J.C., Luo, L., Edo, E.E. et al. Mineralogical Characterization and Optimization of Fe and Mn Through Roast-Leaching of Ferromanganese Ore. Mining, Metallurgy & Exploration 38, 1509–1523 (2021). https://doi.org/10.1007/s42461-021-00390-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00390-2