Abstract
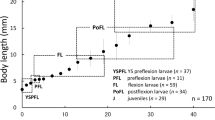
The morphological developments in laboratory-reared larval and juvenile Cirrhinus molitorella and larval C. microlepis were described, and ontogenies (including yolk sizes and absorptions, fin-ray and myomere numbers, allometric changes in the snout, upper jaw, head and pre-anal lengths, maximum body depth, eye diameter and melanophore distributions) were compared. Body lengths (BL) just after hatching were smaller in C. molitorella [3.3 ± 0.1 (mean ± SD) mm] than in C. microlepis (4.4 ± 0.1 mm), and subsequent increase in body lengths were slower in C. molitorella than in C. microlepis. Yolk sizes just after hatching were correspondingly smaller in C. molitorella than in C. microlepis and the yolks were completely absorbed by ca. 95 hours after hatching in the former and ca. 118 hours in the latter. In C. molitorella, fin-rays started appearing at a smaller size than in C. microlepis, with full complements attained by 14.4 mm BL (day 35) in the former, while they did not attain by 16.4 mm BL (day 20) in the latter. Allometric changes started in smaller sizes in C. molitorella than in C. microlepis. Total myomeres were 38–41 in C. molitorella and 39–43 in C. microlepis. Melanophore distributions progressed similarly in both species, except for a darker deposition on the caudal peduncle of C. molitorella of ca. 7–10 mm BL. Larvae of these species were identifiable mainly by differences in fin-ray numbers and allometries by size.








Similar content being viewed by others
References
Allan JD, Abell R, Hogan Z, Revenga C, Taylor BW, Welcomme RL, Winemiller K (2005) Overfishing of inland waters. BioScience 55:1041–1051
Baird I (2011) Cirrhinus microlepis. The IUCN Red List of Threatened Species 2011: e.T180904A765 4985. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T180904A7654985.en. Accessed on 16 September 2020
Baird IG, Inthaphaisy V, Kisouvannalath P, Phylavanh B, Mounsouphom B (1999) The fishes of southern Lao. Lao Community Fisheries and Dolphin Protection Project. Ministry of Agriculture and Forestry, Vientiane
Cacot P, Phengarouni L (2005) Artificial reproduction of the carp Cirrhinus microlepis performed in the South of Laos by using LHRHa implant. In: Burnhil T (ed) Proceedings of 7th technical symposium on Mekong Fisheries. Mekong River Commission, Ubon Ratchathani, pp 171–196
Chea R, Lek S, Ngor P, Grenouillet G (2017) Large-scale patterns of fish diversity and assemblage structure in the longest tropical river in Asia. Ecol Freshw Fish 26:575–585
De Silva SS, Nguyen TTT, Abery NW, Amarasinghe US (2006) An evaluation of the role and impacts of alien finfish in Asian inland aquaculture. Aquac Res 37:1–17
Doi A (1997) A review of taxonomic studies of cypriniform fishes in Southeast Asia. Jpn J Ichthyol 44:1–33
FAO (1997) FAO database on introduced aquatic species. FAO, Rome
Ingram BA, Lasasimma O (2008) Production of Cirrhinus molitorella and Labeo chrysophekadion for culture based fisheries development in Lao PDR Part I: Captive spawning. Aquaculture Asia Magazine: 24–28
Kendall AW Jr, Ahlstrom EH, Moser HG (1984) Early life history stages of fishes and their characters. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL (eds) Ontogeny and systematics of fishes. American Society of Ichthyology and Herpetology Special Publication No 1. Allen Press, Lawrence, pp 11–22
Kottelat M (1998) Fishes of the Nam Theun and Xe Bangfai basins, Laos, with diagnoses of twenty-two new species (Teleostei: Cyprinidae, Balitoridae, Cobitidae, Coiidae and Odontobutidae). Ichthyol Explor Freshw 9:1–128
Kottelat M (2001) Fishes of Laos. Ganaratne Offset Ltd, Colombo
Leis JM, Trnski T (1989) The larvae of Indo-Pacific shorefishes. NSW University Press, Kensington
Lim P, Lek S, Touch ST, Mao SO, Chhouk B (1999) Diversity and spatial distribution of freshwater fish in Great Lake and Tonle Sap River (Cambodia, Southeast Asia). Aquat Living Resour 12:379–386
Monkolprasit SS, Sontirat S, Vimollohakarn S, Songsirikul T (1997) Checklist of fishes in Thailand. Office of Environmental Policy and Planning, Bangkok
Morioka S, Vongvichith B (2011) Growth and morphological development of laboratory-reared larval and juvenile Hemibagrus filamentus (Siluriformes: Bagridae). Ichthyol Res 58:245–254
Morioka S, Ito S, Kitamura S, Vongvichith B (2009) Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus. Ichthyol Res 56:162 – 171
Morioka S, Ito S, Kitamura S (2010a) Growth and morphological development of laboratory-reared larval and juvenile snakeskin gourami Trichogaster pectoralis. Ichthyol Res 57:24–31
Morioka S, Sano K, Phommachan P, Vongvichith B (2010b) Growth and morphological development of laboratory-reared larval and juvenile Pangasianodon hypophthalmus. Ichthyol Res 57:139–147
Morioka S, Cacot P, Moteki M, Thipvantong V, Philavong S, Pounvisouk L, Chantasone P, Thaphysy V (2012a) Ontogenetic development during changeover from an endogenousto exogenous nutritional source in Laotian cyprinid Cirrhinus microlepis larvae. Fish Sci 78:221–227
Morioka S, Chanthasone P, Phommachan P, Vongvichith B (2012b) Growth and morphological development of laboratory-reared larval and juvenile three-spot gourami Trichogaster trichopterus. Ichthyol Res 59:53–62
Morioka S, Vongvichith B, Phommachan P, Chantasone P (2013a) Growth and morphological development of laboratory-reared larval and juvenile bighead catfish Clarias macrocephalus (Siluriformes: Clariidae). Ichthyol Res 60:16–25
Morioka S, Vongvichith B, Phommachan P, Chantasone P (2013b) Growth and morphological development of laboratory-reared larval and juvenile giant gourami Osphronemus goramy (Perciformes: Osphronemidae). Ichthyol Res 60:209–217
Na-Nakorn U, Kamonrat W, Ngamsiric T (2004) Genetic diversity of walking catfish, Clarias macrocephalus, in Thailand and evidence of genetic introgression from introduced farmed C. gariepinus. Aquaculture 240:145–163
Nguyen TTT, De Silva SS (2006) Freshwater finfish biodiversity and conservation: an Asian perspective. Biodivers Conserv 15:3543–3568
Nguyen THT, Van NS, Thinh DV (2011) “Cirrhinus molitorella”. 2011: e.T166016A6168828. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T166016A616882 8.en. Accessed on 16 September 2020
Nuanthavong T, Vilayphone L (2005) Reproduction and nursing of Cirrhinus molitorella in a small fish farm in Luang Prabang Province, Lao PDR. In: Burnhill TJ, Warren TJ (eds) Proceedings of 7th Technical Symposium on Mekong Fisheries, Mekong River Commission, Vientiane, pp 197–203
Ogata Y, Morioka S, Sano K, Vongvichith B, Eda H, Kurokura H, Khonglaliane T (2010) Growth and morphological development of laboratory-reared larvae and juveniles of the Laotioan indigenous cyprinid Hypsibarbus malcolmi. Ichthyol Res 57:389–397
Park JM, Mun SJ, Yim HS, Han KH (2017) Egg development and larvae and juveniles morphology of Carp, Cyprinus carpio in Korean. Dev Reprod 21:287–295
Phillips MJ (2002) Fresh water aquaculture in the Lower Mekong Basin. MRC Technical Paper No 7, Mekong River Commission, Phnom Penh
Poulsen AF, Hortle KG, Valbo-Jorgensen J, Chan S, Chhuon CK, Viravong S, Bouakhamvongsa K, Suntornratana U, Yoorong N, Nguyen TT, Tran BQ (2004) Distribution and ecology of some important riverine fish species of the Mekong River Basin. MRC Technical Paper No 10, Mekong River Commission, Vientiane
Rainboth WJ (1996) Fishes of the Cambodian Mekong. FAO species identification field guide for fishery purposes. FAO, Rome
Roberts TR (1997) Systematic revision of the tropical Asian labeon cyprinid fish genus Cirrhinus, with descriptions of new species and biological observations on C. lobatus. Nat Hist Bull Siam Soc 45:171–203
Santos JNS, Araújo FG, Silva DS (2009) Length correction for early-juvenile Brazilian herring Sardinella janeiro (Eigenmann, 1894) after preservation in formalin, ethanol and freezing. Neotrop Ichthyol 7:87–92
Senanan W, Kapuscinski AR, Na-Nakorn U, Miller LM (2004) Genetic impacts of hybrid catfish farming (Clarias macrocephalus × C. gariepinus) on native catfish populations in central Thailand. Aquaculture 235:167–184
Shield PA, Carlson SR (1998) Effects of formalin and alchol preservation on lengths and weights of juvenile Sockeye salmon. Alsk Fish Res Bull 3:81–93
Singhanouvong DC, Soulignavong K, Vonghachak B, Saadsy, Warren TJ (1996) The main dry-season fish migrations of the Mekong mainstream at Hat Village, Muang Khong District, Hee Village, Muang Mouan District and Hatsalao Village, Paxse. Indigenous Fishery Development Project, Fisheries Ecology Technical Report No 3, Vientiane
Sverdrup-Jensen S (2002) Fisheries in the lower Mekong basin: status and perspectives. MRC Technical Paper No 6, Mekong River Commission, Phnom Penh
Termvidchakorn A, Hortle KG (2013) A guide to larvae and juveniles of some common fish species from the Mekong River Basin. MRC Technical Paper No 38, Mekong River Commission, Phnom Penh
Welcomme RL, Vidthayanon C (2003) The impact of introductions and stocking of exotic species in the Mekong basin and policies for their control. MRC Technical Paper No 9, Mekong River Commission, Phnom Penh
Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S, Baird IG, Darwall W, Lujan NK, Harrison I, Stiassny MLJ, Silvano RAM, Fitzgerald DB, Pelicice FM, Agostinho AA, Gomes LC, Albert JS, Baran E, Petrere M, Zarfl C, Mulligan M, Sullivan JP, Arantes CC, Sousa LM, Koning AA, Hoeinghaus DJ, Sabaj M, Lundberg JG, Armbruster J, Thieme ML, Petry P, Zuanon J, Torrente Vilara S, Ou C, Rainboth W, Pavanelli CS, Akama A, van Soesbergen A, Sáenz L (2016) Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong: Basin-scale planning is needed to minimize impacts in mega-diverse rivers. Science 351:128–129
Acknowledgements
We express our sincere gratitude to Masuo Ando, Utsunomiya University, Kazuyuki Matsuo, Shizuoka Professional University of Agriculture, Masayoshi Saito, Yukiyo Yamamoto and Osamu Abe, Japan International Research Center for Agricultural Sciences, for their helpful coordination of the research activity. Our appreciations also go to the technical staff of the Living Aquatic Resources Research Center, Laos, for their cooperative assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Morioka, S.M., Vongvichith, B., Chanthasone, P. et al. Developmental morphology and growth in early stages of laboratory-reared Cirrhinus molitorella and C. microlepis (Cypriniformes: Cyprinidae). Ichthyol Res 68, 506–516 (2021). https://doi.org/10.1007/s10228-021-00803-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-021-00803-8