Abstract
Introduction
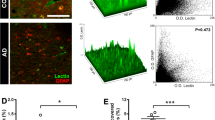
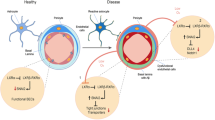
Cell chirality is an intrinsic cellular property that determines the directionality of cellular polarization along the left–right axis. We recently show that endothelial cell chirality can influence intercellular junction formation and alter trans-endothelial permeability, depending on the uniformity of the chirality of adjacent cells, which suggests a potential role for cell chirality in neurodegenerative diseases with blood–brain barrier (BBB) dysfunctions, such as Alzheimer’s disease (AD). In this study, we determined the effects of AD-related proteins amyloid-β (Aβ), tau, and apolipoprotein E4 (ApoE4) on the chiral bias of the endothelial cell component in BBB.
Methods
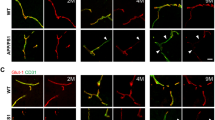
We first examined the chiral bias and effects of protein kinase C (PKC)-mediated chiral alterations of human brain microvascular endothelial cells (hBMECs) using the ring micropattern chirality assay. We then investigated the effects of Aβ, tau, and ApoE4 on hBMEC chirality using chirality assay and biased organelle positions.
Results
The hBMECs have a strong clockwise chiral bias, which can be reversed by protein kinase C (PKC) activation. Treatment with tau significantly disrupted the chiral bias of hBMECs with altered cellular polarization. In contrast, neither ApoE4 nor Aβ-42 caused significant changes in cell chirality.
Conclusions
We conclude that tau might cause BBB dysfunction by disrupting cell polarization and chiral morphogenesis, while the effects of ApoE4 and Aβ-42 on BBB integrity might be chirality-independent. The potential involvement of chiral morphogenesis in tau-mediated BBB dysfunction in AD provides a novel perspective in vascular dysfunction in tauopathies such as AD, chronic traumatic encephalopathy, progressive supranuclear palsy, and frontotemporal dementia.




Similar content being viewed by others
References
Barre, P., and D. Eliezer. Structural transitions in tau k18 on micelle binding suggest a hierarchy in the efficacy of individual microtubule-binding repeats in filament nucleation. Protein Sci. 22:1037–1048, 2013.
Cabrales Fontela, Y., H. Kadavath, J. Biernat, D. Riedel, E. Mandelkow, and M. Zweckstetter. Multivalent cross-linking of actin filaments and microtubules through the microtubule-associated protein Tau. Nat. Commun. 8(1):1–12, 2017. https://doi.org/10.1038/s41467-017-02230-8.
Camici, G. G., G. Savarese, A. Akhmedov, and T. F. Lüscher. Molecular mechanism of endothelial and vascular aging: Implications for cardiovascular disease. Eur. Heart J. 36:3392–3403, 2015.
Chen, J., Y. Kanai, N. J. Cowan, and N. Hirokawa. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 360:674–677, 1992.
Chin, A. S., et al. Epithelial cell chirality revealed by three-dimensional spontaneous rotation. Proc. Natl. Acad. Sci. U. S. A. 115:12188–12193, 2018.
Cleveland, D. W., S. Y. Hwo, and M. W. Kirschner. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 116:207–225, 1977.
Di Marco, L. Y., et al. Vascular dysfunction in the pathogenesis of Alzheimer’s disease: a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 82:593–606, 2015.
Drake, J., C. D. Link, and D. A. Butterfield. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 24:415–420, 2003.
Drechsel, D. N., A. A. Hyman, M. H. Cobb, and M. W. Kirschner. Drechsel. Mol. Biol. Cell 3:1141–1154, 1992.
Elie, A., et al. Tau co-organizes dynamic microtubule and actin networks. Sci. Rep. 5:1–10, 2015.
Fan, J., H. Zhang, T. Rahman, D. N. Stanton, and L. Q. Wan. Cell organelle-based analysis of cell chirality. Commun. Integr. Biol. 12:78–81, 2019.
Fan, J., et al. Cell chirality regulates intercellular junctions and endothelial permeability. Sci. Adv. 4:2111, 2018.
Fulga, T. A., I. Elson-Schwab, V. Khurana, M. L. Steinhilb, T. L. Spires, B. T. Hyman, and M. B. Feany. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat. Cell Biol. 9(2):139–148, 2007. https://doi.org/10.1038/ncb1528.
Gonzalez-Velasquez, F. J., J. A. Kotarek, and M. A. Moss. Soluble aggregates of the amyloid-β protein selectively stimulate permeability in human brain microvascular endothelial monolayers. J. Neurochem. 107:466–477, 2008.
Henríquez, J. P., D. Cross, C. Vial, and R. B. Maccioni. Subpopulations of tau interact with microtubules and actin filaments in various cell types. Cell Biochem. Funct. 13(4):239–250, 1995. https://doi.org/10.1002/cbf.290130404.
Hoelzle, M. K., and T. Svitkina. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol. Biol. Cell 23:310–323, 2012.
Holtzman, D. M. Vivo effects of ApoE and clusterin on amyloid- β metabolism and neuropathology evidence that amyloid- β conformational to Alzheimer’ s disease. Cell Biol. 23:247–254, 2004.
Hu, Y., X. Yao, Q. Liu, Y. Wang, R. Liu, S. Cui, and J. Ding. Left-right symmetry or asymmetry of cells on stripe-like micropatterned material surfaces. Chin. J. Chem. 36(7):605–611, 2018. https://doi.org/10.1002/cjoc.201800124.
Jiang, Q., et al. ApoE promotes the proteolytic degradation of Aβ. Neuron 58:681–693, 2008.
Kovac, A., M. Zilkova, M. A. Deli, N. Zilka, and M. Novak. Human truncated tau is using a different mechanism from amyloid-β to damage the blood-brain barrier. J. Alzheimer’s Dis. 18:897–906, 2009.
Larsson, C. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18(3):276–284, 2006. https://doi.org/10.1016/j.cellsig.2005.07.010.
Lee, W., et al. Amyloid beta peptide directly inhibits PKC activation. Mol. Cell. Neurosci. 26:222–231, 2004.
Leszek, J., M. Sochocka, and K. Gasiorowski. Vascular factors and epigenetic modifications in the pathogenesis of Alzheimer’s disease. J. Neurol. Sci. 323:25–32, 2012.
Maoz, B. M., et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 36:865–877, 2018.
Marco, S., and S. D. Skaper. Amyloid β-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci. Lett. 401:219–224, 2006.
Nishitsuji, K., T. Hosono, T. Nakamura, G. Bu, and M. Michikawa. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 286:17536–17542, 2011.
Olsson, B., et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15:673–684, 2016.
Polacheck, W. J., et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552:258–262, 2017.
Ray, P., et al. Intrinsic cellular chirality regulates left–right symmetry breaking during cardiac looping. Proc. Natl. Acad. Sci. U. S. A. 115:E11568–E11577, 2018.
Sandoval, R., A. B. Malik, R. D. Minshall, P. Kouklis, C. A. Ellis, and C. Tiruppathi. Ca2+ signalling and PKCα activate increased endothelial permeability by disassembly of VE-cadherin junctions. J. Physiol. 533(2):433–445, 2001. https://doi.org/10.1111/j.1469-7793.2001.0433a.x.
Satoh, J. I., Y. Kino, and S. Niida. MicroRNA-Seq data analysis pipeline to identify blood biomarkers for alzheimer’s disease from public data. Biomark. Insights 2015:21–31, 2015.
Stopschinski, B. E., et al. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus -synuclein and -amyloid aggregates. J. Biol. Chem. 293:10826–10840, 2018.
Sullivan, K. G., L. N. Vandenberg, and M. Levin. Cellularmigration may exhibit intrinsic left-right asymmetries: a meta-analysis. bioRxiv. 2018:269217.
Sweeney, M. D., A. P. Sagare, and B. V. Zlokovic. Blood–brain barrier breakdown in Alzheimer’s disease and other pdf. Nat. Rev. Neurol. 14:133–150, 2018.
Tamada, A., and M. Igarashi. Revealing chiral cell motility by 3D Riesz transform-differential interference contrast microscopy and computational kinematic analysis. Nat. Commun. 2017. https://doi.org/10.1038/s41467-017-02193-w.
Tee, Y. H., et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol. 17:445–457, 2015.
Tokuda, T., et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid β peptides. Biochem. J. 348:359–365, 2000.
Trinczek, B., J. Biernat, K. Baumann, E. Mandelkow, and E. M. Mandelkow. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol. Biol. Cell 6:1887–1902, 1995.
Verheijen, J., and K. Sleegers. Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet. 34:434–447, 2018.
Wan, L. Q., A. S. Chin, K. E. Worley, and P. Ray. Cell chirality: emergence of asymmetry from cell culture. Philos. Trans. R. Soc. B Biol. Sci. 371:20150413, 2016.
Wan, L. Q., et al. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc. Natl. Acad. Sci. 108:12295–12300, 2011.
Weingarten, M. D., A. H. Lockwood, S. Y. Hwo, and M. W. Kirschner. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. U. S. A. 72:1858–1862, 1975.
Xu, Q., et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J. Neurosci. 26:4985–4994, 2006.
Yao, X., and J. Ding. Effects of microstripe geometry on guided cell migration. ACS Appl. Mater. Interfaces. 12(25):27971–27983, 2020. https://doi.org/10.1021/acsami.0c05024.
Ye, M., et al. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 4:1–6, 2014.
Zhao, J., et al. 3-O-Sulfation of heparan sulfate enhances tau interaction and cellular uptake. Angew. Chemie - Int. Ed. 59:1818–1827, 2020.
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12:723–738, 2011.
Acknowledgments
This work was funded by the National Institutes of Health (OD/NICHD DP2HD083961). Leo Q. Wan is a Pew Scholar in Biomedical Sciences (PEW 00026185), supported by the Pew Charitable Trusts.
Conflict of interest
Haokang Zhang, Jie Fan, Zhen Zhao, Chunyu Wang and Leo Q. Wan declare that they have no conflicts of interest.
Ethical standards
No human studies or animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Daniel Fletcher oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Fan, J., Zhao, Z. et al. Effects of Alzheimer’s Disease-Related Proteins on the Chirality of Brain Endothelial Cells. Cel. Mol. Bioeng. 14, 231–240 (2021). https://doi.org/10.1007/s12195-021-00669-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00669-w