Abstract
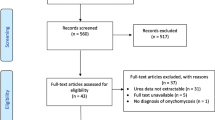
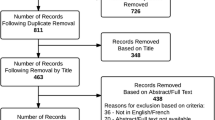
Fosravuconazole L-lysine ethanolate (F-RVCZ), a ravuconazole prodrug, is a newly available agent with high expectations for efficacy in the treatment of onychomycosis. However, clinical data regarding the efficacy of F-RVCZ are limited because the drug was launched only in Japan in 2018. Therefore, we analyzed the outcome of F-RVCZ therapy in the treatment of onychomycosis at outpatient dermatology clinics in Japan. We examined data for 109 patients (68 male, 41 female) with varying clinical type, including total dystrophic onychomycosis and dermatophytoma, and a wide range of age groups, including the elderly. The complete cure rate at 12 weeks was 6.4% (7/109) and 67.9% (74/109) at the last visit (mean time to last visit: 32 ± 14.2 weeks). Mean rate of improvement in the affected nail area was 49.1 ± 23.3% at 12 weeks and 86.8 ± 22.4% at the last visit. Efficacy at 12 weeks and the last visit, respectively, was as follows: none, 4 cases and 1 case; slight, 35 cases and 4 cases; moderate, 51 cases and 21 cases; significant, 12 cases and 9 cases; complete cure, 7 cases and 74 cases. There were no serious adverse events. This retrospective survey was the first large-scale analysis of actual clinical practice outcomes and had minimal exclusions. Compared to previous reports, our results demonstrated excellent efficacy of F-RVCZ therapy in a variety of patients. Considering our results and the ease of oral administration (1 capsule/day for 12 weeks) and few adverse events, F-RVCZ therapy appears to be a useful option for the treatment of onychomycosis.

Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st century: an update on diagnosis, epidemiology, and treatment. J Cutan Med Surg. 2017;21(6):525–39.
Gupta AK, Jain HC, Lynde CW, Macdonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15 000 patients. J Am Acad Dermatol. 2000;43(2):244–8.
Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36(10):754–6.
Wang J, Wiznia LE, Rieder EA. Patient-reported outcomes in onychomycosis: a review of psychometrically evaluated instruments in assessing treatment effectiveness. Skin Appendage Disord. 2017;3(3):144–55.
Ameen M, Lear JT, Madan V, Mohd Mustapa MF, Richardson M. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br J Dermatol. 2012;171(5):937–58.
Seebacher C, Brasch J, Abeck D, Cornely O, Effendy I, Ginter-Hanselmayer G, et al. Onychomycosis. Mycoses. 2007;50(4):321–7.
Mochizuki T, Tsuboi R, Iozumi K, Ishizaki S, Ushigami T, Ogawa Y, et al. Guidelines for the management of dermatomycosis (2019). J Dermatol. 2020;47(12):1343–73.
Iozumi K, Abe M, Ito Y, Uesugi T, Onoduka T, Ichiro Kato I, et al. Efficacy of long-term treatment with efinaconazole 10% solution in patients with onychomycosis, including severe cases: a multicenter, single-arm study. J Dermatol. 2019;46(8):641–51.
Watanabe S, Kishida H, Okubo A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2017;44(7):753–9.
Shimoyama H, Kuwano Y, Sei Y. Retrospective survey of treatment outcomes of efinaconazole 10% solution and luliconazole 5% solution for onychomycosis in our facility. Med Mycol J. 2019;60(4):95–100.
Yamaguchi H. Potential of ravuconazole and its prodrugs as the new oral therapeutics for onychomycosis. Med Mycol J. 2016;57(4):E93–110.
Gupta AK, Leonardi C, Stoltz RR, Pierce PF, Conetta B. Ravuconazole Onychomycosis Group A phase I/II randomized, doubleblind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(4):437–43.
Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45(10):1151–9.
Cuenca-Estrella M, Gomez-Lopez A, Mellado E, Garcia-Effron G, Rodriguez-Tudela JL. In vitro activities of ravuconazole and four other antifungal agents against fluconazole-resistant or -susceptible clinical yeast isolates. Antimicrob Agents Chemother. 2004;48(8):3107–11.
Noguchi H, Matsumoto T, Kimura U, Hiruma M, Kano R, Kubo M, et al. 2020 Fosravuconazole to treat severe onychomycosis in the elderly. J Dermatol doi: https://doi.org/10.1111/1346-8138.15651. Online ahead of print.
Martinez-Herrera E, Moreno-Coutiño G, Fernández-Martínez RF, Finch J, Arenas R. Dermatophytoma: description of 7 cases. J Am Acad Dermatol. 2012;66:1014–6.
Burkhart CN, Burkhart CG, Gupta AK. Dermatophytoma: recalcitrance to treatment because of existence of fungal biofilm. J Am Acad Dermatol. 2002;47(4):629–31.
Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification. J Am Acad Dermatol. 2011;65(6):1219–27.
Shimoyama H, Sei Y. 2016 Epidemiological Survey of Dermatomycoses in Japan. Med Mycol J. 2019;60(3):75–82.
Loo DS. Onychomycosis in the elderly: drug treatment options. Drugs Aging. 2007;24(4):293–302.
Reszke R, Pełka D, Walasek A, Machaj Z, Reich A. Skin disorders in elderly subjects. Int J Dermatol. 2015;54(9):e332-338.
Sigurgeirsson B, Billstein S, Rantanen T, Ruzicka T, di Fonzo E, Vermeer BJ, et al. LION Study: efficacy and tolerability of continuous terbinafine (Lamisil) compared to intermittent itraconazole in the treatment of toenail onychomycosis Lamisil versus Itraconazole in Onychomycosis. Br J Dermatol. 1999;141(56):5–14.
Takeshima R, Asahina Y, Yaguchii T, Sato T. Tinea barbae due to Trichophyton rubrum successfully treated using oral fosravuconazole l-lysine ethanolate. J Dermatol. 2020;47(7):e254–5.
Noguchi H, Matsumoto T, Kimura U, Hiruma M, Kano R, Yaguchi T, et al. Fungal melanonychia caused by Candida parapsilosis successfully treated with oral fosravuconazole. J Dermatol. 2019;46(10):911–3.
Noguchi H, Matsumoto T, Hiruma M, Kimura U, Kano R, Yaguchi T, et al. Tinea unguium caused by terbinafine-resistant Trichophyton rubrum successfully treated with fosravuconazole. J Dermatol. 2019;46(12):e446–7.
Acknowledgements
The authors thank Koichi Makimura of Teikyo University for contributions based on expertise in mycology. The authors thank Kazuo Satoh of Teikyo University for contributions to the statistical analyses and manuscript revisions.
Author information
Authors and Affiliations
Contributions
HS designed the study, carried out the diagnosis, treatment, and data analysis, and drafted the manuscript. YS, AY, and YK participated in the diagnosis and treatment of onychomycosis and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent for publication was obtained from the study participant presented in Fig. 1.
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committees (Research Ethics Committee of Teikyo University; Tei-rin 18–235-2) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Rui Kano.
Rights and permissions
About this article
Cite this article
Shimoyama, H., Yo, A., Sei, Y. et al. Treatment Outcome with Fosravuconazole for Onychomycosis. Mycopathologia 186, 259–267 (2021). https://doi.org/10.1007/s11046-021-00540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-021-00540-6