Abstract
Background and aim
As a proinflammatory cytokine, tumor necrosis factor-like weak inducer of apoptosis (TWEAK) participates in the progression of renal fibrosis by binding to its receptor, fibroblast growth factor-inducible 14 (Fn14). However, the effect of Fn14 inhibition on tubular epithelial cell-mediated tubulointerstitial fibrosis remains unclear. This study aimed to elucidate the role of TWEAK/Fn14 interaction in the development of experimental tubulointerstitial fibrosis as well as the protective effect of Fn14 knockdown on proximal tubular epithelial cells.
Methods
A murine model of unilateral ureteral obstruction was constructed in both wild-type and Fn14-deficient BALB/c mice, followed by observation of the tubulointerstitial pathologies.
Results
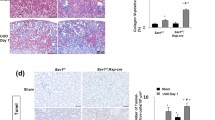
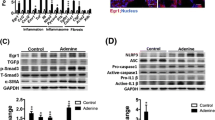
Fn14 deficiency ameliorated the pathological changes, including inflammatory cell infiltration and cell proliferation, accompanied by reduced production of profibrotic factors and extracellular matrix deposition. In vitro experiments showed that TWEAK dose-dependently enhanced the expression of collagen I, fibronectin, and α-smooth muscle actin in proximal tubular epithelial cells. Interestingly, TWEAK also upregulated the expression levels of Notch1/Jagged1. Fn14 knockdown and Notch1/Jagged1 inhibition also mitigated the effect of TWEAK on these cells.
Conclusions
In conclusion, TWEAK/Fn14 signals contributed to tubulointerstitial fibrosis by acting on proximal tubular epithelial cells. Fn14 inhibition might be a therapeutic strategy for protecting against renal interstitial fibrosis.








Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Ucero AC, Benito-Martin A, Fuentes-Calvo I, Santamaria B, Blanco J, Lopez-Novoa JM, Ruiz-Ortega M, Egido J, Burkly LC, Martinez-Salgado C. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta. 2013;1832:1744–55.
Sanz AB, Sanchez-Nino MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80:708–18.
Chen JY, Wei LL, Xia YM. Roles of tumour necrosis factor-related weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway in lupus nephritis. Nephrology (Carlton). 2017;22:101–6.
Weinberg JM. TWEAK-Fn14 as a mediator of acute kidney injury. Kidney Int. 2011;79:151–3.
Mo SJ, Zhang W, Liu JQ, Chen MH, Xu L, Hong J, Li Q, Yang XH, Sun RH, Hu BC. Regulation of Fn14 stability by SCFFbxw7α during septic acute kidney injury. Am J Physiol Renal Physiol. 2019;316:F1273–81.
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–58.
Burkly LC. TWEAK/Fn14 axis: the current paradigm of tissue injury-inducible function in the midst of complexities. Semin Immunol. 2014;26:229–36.
Liu J, Liu YL, Peng LL, Li JX, Wu KY, Xia LL, Wu JW, Wang SJ, Wang XN, Liu QL, Zeng WH, Xia YM. TWEAK/Fn14 signals mediate burn wound repair. J Invest Dermatol. 2019;139:224–34.
Schnaper HW. The tubulointerstitial pathophysiology of progressive kidney disease. Adv Chronic Kidney Dis. 2017;24:107–16.
Gewin LS. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018;68–69:248–62.
Hong WL, Zhang G, Lu H, Guo YY, Zheng SZ, Zhu HY, Xiao YY, Papa A, Wu CZ, Sun LX, Chen BC, Bai YH. Epithelial and interstitial Notch1 activity contributes to the myofibroblastic phenotype and fibrosis. Cell Commun Signal. 2019;17:145.
Xia YM, Campbell SR, Broder A, Herlitz L, Abadi M, Wu P, Michaelson JS, Burkly LC, Putterman C. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol. 2012;145:108–21.
Xia YM, Herlitz LC, Gindea S, Wen J, Pawar RD, Misharin A, Perlman H, Wu L, Wu P, Michaelson JS, Burkly LC, Putterman C. Deficiency of fibroblast growth factor-inducible 14 (Fn14) preserves the filtration barrier and ameliorates lupus nephritis. J Am Soc Nephrol. 2015;26:1053–70.
Peng L, Li Q, Wang H, Wu J, Li C, Liu Y, Liu J, Xia L, Xia Y. Fn14 deficiency ameliorates psoriasis-like skin disease in a murine model. Cell Death Dis. 2018;9:801.
Fierro-Fernández M, Miguel V, Márquez-Expósito L, Nuevo-Tapioles C, Herrero JI, Blanco-Ruiz E, Tituana J, Castillo C, Cannata P, Monsalve M, Ruiz Ortega M, Ramos R, Lamas S. MiR-9-5p protects from kidney fibrosis by metabolic reprogramming. FASEB J. 2020;34:410–31.
Choudhry N, Li K, Zhang T, Wu KY, Song Y, Farrar CA, Wang N, Liu CF, Peng Q, Wu W, Sacks SH, Zhou WD. The complement factor 5a receptor1 has a pathogenic role in chronic inflammation and renal fibrosis in a murine model of chronic pyelonephritis. Kidney Int. 2016;90:540–54.
Liu YL, Peng LL, Li L, Liu CF, Hu X, Xiao SX, Xia YM. TWEAK/Fn14 activation contributes to the pathogenesis of bullous pemphigoid. J Invest Dermatol. 2017;137:1512–22.
Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–306.
Soni H, Matthews AT, Pallikkuth S, Gangaraju R, Adebiyi A. γ-secretase inhibitor DAPT mitigates cisplatin-induced acute kidney injury by suppressing Notch1 signaling. J Cell Mol Med. 2019;23:260–70.
Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, Nakamura S, Konishi N, Yagita H, Nonomura K, Nakajima Y. Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int. 2011;79:179–88.
Gomez IG, Roach AM, Nakagawa N, Amatucci A, Johnson BG, Dunn K, Kelly MC, Karaca G, Zheng TS, Szak S, Peppiatt-Wildman CM, Burkly LC, Duffield JS. TWEAK-Fn14 signaling activates myofibroblasts to drive progression of fibrotic kidney disease. J Am Soc Nephrol. 2016;27:3639–52.
Wang YH, Zhou QL, Tang R, Huang YY, He T. FoxM1 inhibition ameliorates renal interstitial fibrosis by decreasing extracellular matrix and epithelial-mesenchymal transition. J Pharmacol Sci. 2020;143:281–9.
Li NN, Wang Z, Gao FL, Lei YF, Li ZZ. Melatonin ameliorates renal fibroblast-myofibroblast transdifferentiation and renal fibrosis through miR-21-5p regulation. J Cell Mol Med. 2020;24:5615–28.
Mohamed R, Jayakumar C, Ramesh G. Chronic administration of EP4-selective agonist exacerbates albuminuria and fibrosis of the kidney in streptozotocin-induced diabetic mice through IL-6. Lab Invest. 2013;93:933–45.
Chen JY, Jia FY, Ren KX, Luo M, Min XY, Wang P, Xiao SX, Xia YM. Inhibition of suppressor of cytokine signaling 1 mediates the profibrotic effect of TWEAK/Fn14 signaling on kidney cells. Cell Signal. 2020;71:109615.
Wang HX, Wang JX, Xia YM. Defective suppressor of cytokine signaling 1 signaling contributes to the pathogenesis of systemic lupus erythematosus. Front Immunol. 2017;8:1292.
Wang YY, Jiang H, Pan J, Huang XR, Wang YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY, Chen JH. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28:2053–67.
Gu TT, Chen TY, Yang YZ, Zhao XJ, Sun Y, Li TS, Zhang DM, Kong LD. Pterostilbene alleviates fructose-induced renal fibrosis by suppressing TGF-β1/TGF-β type I receptor/Smads signaling in proximal tubular epithelial cells. Eur J Pharmacol. 2019;842:70–8.
Burkly LC. Regulation of tissue responses: the TWEAK/Fn14 pathway and other tnf/tnfr superfamily members that activate non-canonical NFκB signaling. Front Immunol. 2015;6:92.
Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–54.
Funding
This study was supported by the National Natural Science Foundation of China (Project No.81874241) and the Innovation Capability Support Plan of Shaanxi Province (No.2019TD-034).
Author information
Authors and Affiliations
Contributions
Mai Luo and Mengmeng Liu participated in the design of the study, and performed most experimental work. WL, XC, SZ, HG, HW, and KW carried out some experiments. WZ discussed the experimental data and contributed to the interpretation of results. KL and YX conceived and designed the study and prepared the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was carried out in accordance with the recommendations of the guidelines of the University Research Ethics Committee. These protocols were approved by the University Research Ethics Committee.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2021_1455_MOESM1_ESM.tif
Supplementary file1 Fig. S1 Efficiency of siRNA transfection in HK2 cells. HK2 cells were cultured in vitro and transfected with Fn14 or control siRNA. a Efficiency of siRNA transfection assessed by confocal microscopy. b The Fn14 mRNA expression levels were assessed via qRT-PCR. c and d Western blot analysis revealed the Fn14 protein expression levels in the cell lysates. Data were obtained from three independent experiments. Representative images are shown. **P < 0.01. ns, Not significant (TIF 693 KB)
11_2021_1455_MOESM2_ESM.tif
Supplementary file2 Fig. S2 Appearance and histology changes after unilateral ureteral obstruction (UUO). a The obstructed kidney (left) and nonobstructed kidney (right) were isolated after UUO (7days). b H&E staining showing histological changes on day 7; arrows indicate injured tubules and inflammatory cell infiltration (TIF 2343 KB)
11_2021_1455_MOESM3_ESM.tif
Supplementary file3 Fig. S3 TWEAK increased proinflammatory factor production in HK2 cells. HK2 cells were cultured in vitro and stimulated with recombinant TWEAK (0–250 ng/mL) for 24 h. mRNA expression levels of IL-6 (a), TNF-α (b), and MCP-1 (c) were assessed in cells via qRT-PCR. Data were obtained from three independent experiments. *P < 0.05, **P < 0.01, compared with the blank group (TIF 870 KB)
11_2021_1455_MOESM4_ESM.tif
Supplementary file4 Fig. S4 The efficiency of Fn14 deficiency in BALB/c mice determined by Western blot analysis (TIF 335 KB)
Rights and permissions
About this article
Cite this article
Luo, M., Liu, M., Liu, W. et al. Inhibition of fibroblast growth factor-inducible 14 attenuates experimental tubulointerstitial fibrosis and profibrotic factor expression of proximal tubular epithelial cells. Inflamm. Res. 70, 553–568 (2021). https://doi.org/10.1007/s00011-021-01455-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01455-0