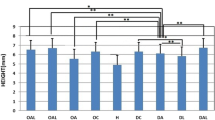
Abstract
Current treatment options for osteoporosis primarily involve pharmacotherapies, but they are often accompanied by undesirable side effects. Utilization of mechanical stress which can noninvasively induce bone formation has been suggested as an alternative to conventional treatments. Here, we examined the efficacy of mechanical stress induced by electrical stimulation, radial extracorporeal shock waves, and ultrasound for estrogen-deficient osteoporosis. Female Wistar rats were divided into following five groups: sham-operated group, untreated after ovariectomy, and treated with electrical stimulation, radial extracorporeal shock wave, or ultrasound starting at 8 weeks after ovariectomy for 4 weeks. Trabecular bone architecture of the femur was assessed by micro-CT and its biomechanical properties were obtained by mechanical testing. The femurs were further evaluated by histochemical, immunohistochemical, and real-time PCR analyses. Radial extracorporeal shock wave and ultrasound treatment improved trabecular bone microarchitecture and bone strength in osteoporotic rats, but not electrical stimulation. The shock wave decreased osteoclast activity and RANKL expression. The exposure of ultrasound increased osteoblast activity and β-catenin-positive cells, and they decreased sclerostin-positive osteocytes. These findings suggest that mechanical stress induced by radial extracorporeal shock wave and ultrasound can improve estrogen-deficient bone loss and bone fragility through promoted bone formation or attenuated bone resorption.






Similar content being viewed by others
Data Availability
The datasets used and analyzed during this study are available from corresponding author upon request.
Change history
25 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00223-021-00867-8
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 285(6):785–795
Lips P, van Schoor NM (2005) Quality of life in patients with osteoporosis. Osteoporos Int 16:447–455. https://doi.org/10.1007/s00198-004-1762-7
Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, Santora AC et al (2000) Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. J Am Geriatr Soc 48:241–249. https://doi.org/10.1111/j.1532-5415.2000.tb02641.x
McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20. https://doi.org/10.1016/j.amjmed.2012.06.023
Bilezikian JP (2006) Osteonecrosis of the jaw - do bisphosphonates pose a risk? N Engl J Med 355:2278–2281. https://doi.org/10.1056/NEJMp068157
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) Physical activity and bone health. Med Sci Sports Exerc 36:1985–1996. https://doi.org/10.1249/01.MSS.0000142662.21767.58
Moreira LDF, de Oliveira ML, Lirani-Galvão AP, Marin-Mio RV, dos Santos RN, Lazaretti-Castro M (2014) Physical exercise and osteoporosis: effects of different types of exercises on bone and physical function of postmenopausal women. Arq Bras Endocrinol Metabol 58:514–522. https://doi.org/10.1590/0004-2730000003374
Duncan RL, Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57:344–358. https://doi.org/10.1007/BF00302070
Turner CH (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407. https://doi.org/10.1016/S8756-3282(98)00118-5
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM et al (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875. https://doi.org/10.1074/jbc.M705092200
Lam H, Qin Y-X (2008) The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone 43:1093–1100. https://doi.org/10.1016/j.bone.2008.07.253
Midura RJ, Dillman CJ, Grabiner MD (2005) Low amplitude, high frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med Eng Phys 27:285–293. https://doi.org/10.1016/j.medengphy.2004.12.014
Jee WS, Yao W (2001) Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1:193–207
Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH (2004) Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone 34:225–230. https://doi.org/10.1016/j.bone.2003.08.005
Rompe JD, Rosendahl T, Schöllner C, Theis C (2001) High-energy extracorporeal shock wave treatment of nonunions. Clin Orthop Relat Res. https://doi.org/10.1097/00003086-200106000-00014
Van Der Jagt OP, Van Der Linden JC, Schaden W, Van Schie HT, Piscaer TM, Verhaar JAN, Weinans H et al (2009) Unfocused extracorporeal shock wave therapy as potential treatment for osteoporosis. J Orthop Res 27:1528–1533. https://doi.org/10.1002/jor.20910
Van Der Jagt OP, Waarsing JH, Kops N, Schaden W, Jahr H, Verhaar JAN, Weinans H (2013) Unfocused extracorporeal shock waves induce anabolic effects in osteoporotic rats. J Orthop Res 31:768–775. https://doi.org/10.1002/jor.22258
Koolen MKE, Kruyt MC, Öner FC, Schaden W, Weinans H, van der Jagt OP (2019) Effect of unfocused extracorporeal shockwave therapy on bone mineral content of twelve distal forearms of postmenopausal women: a clinical pilot study. Arch Osteoporos. https://doi.org/10.1007/s11657-019-0650-x
Watanabe Y, Matsushita T, Bhandari M, Zdero R, Schemitsch EH (2010) Ultrasound for fracture healing: current evidence. J Orthop Trauma 24:S56–S61. https://doi.org/10.1097/BOT.0b013e3181d2efaf
Lim D, Ko CY, Seo DH, Woo DG, Kim JM, Chun KJ, Kim HS (2011) Low-intensity ultrasound stimulation prevents osteoporotic bone loss in young adult ovariectomized mice. J Orthop Res 29:116–125. https://doi.org/10.1002/jor.21191
Ferreri SL, Talish R, Trandafir T, Qin Y-X (2011) Mitigation of bone loss with ultrasound induced dynamic mechanical signals in an OVX induced rat model of osteopenia. Bone 48:1095–1102. https://doi.org/10.1016/j.bone.2011.01.002
Warden SJ, Bennell KL, Forwood MR, McMeeken JM, Wark JD (2001) Skeletal effects of low-intensity pulsed ultrasound on the ovariectomized rodent. Ultrasound Med Biol 27:989–998. https://doi.org/10.1016/S0301-5629(01)00376-3
Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD (2001) Efficacy of low-intensity pulsed ultrasound in the prevention of osteoporosis following spinal cord injury. Bone 29:431–436. https://doi.org/10.1016/S8756-3282(01)00599-3
Leung KS, Lee WS, Cheung WH, Qin L (2004) Lack of efficacy of low-intensity pulsed ultrasound on prevention of postmenopausal bone loss evaluated at the distal radius in older Chinese women. Clin Orthop Relat Res. https://doi.org/10.1097/01.blo.0000137557.59228.4d
Angle SR, Sena K, Sumner DR, Virdi AS (2011) Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics 51:281–288. https://doi.org/10.1016/j.ultras.2010.09.004
Li JGR, Chang WHS, Lin JCA, Sun JS (2002) Optimum intensities of ultrasound for PGE2 secretion and growth of osteoblasts. Ultrasound Med Biol 28:683–690. https://doi.org/10.1016/S0301-5629(02)00485-4
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191. https://doi.org/10.1016/0169-6009(91)90124-I
Stürmer EK, Seidlová-Wuttke D, Sehmisch S, Rack T, Wille J, Frosch KH, Wuttke W et al (2006) Standardized bending and breaking test for the normal and osteoporotic metaphyseal tibias of the rat: Effect of estradiol, testosterone, and raloxifene. J Bone Miner Res 21:89–96. https://doi.org/10.1359/JBMR.050913
Kawamoto T (2003) Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol 66:123–143. https://doi.org/10.1679/aohc.66.123
McKenzie J, Smith C, Karuppaiah K, Langberg J, Silva MJ, Ornitz DM (2019) Osteocyte death and bone overgrowth in mice lacking fibroblast growth factor receptors 1 and 2 in mature osteoblasts and osteocytes. J Bone Miner Res 34:1660–1675. https://doi.org/10.1002/jbmr.3742
Moriyama H, Kanemura N, Brouns I, Pintelon I, Adriaensen D, Timmermans JP, Ozawa J et al (2012) Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology 13:369–381. https://doi.org/10.1007/s10522-012-9381-8
Nomura M, Sakitani N, Iwasawa H, Kohara Y, Takano S, Wakimoto Y, Kuroki H et al (2017) Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthr Cartil 25:727–736. https://doi.org/10.1016/j.joca.2016.11.013
Kaneguchi A, Ozawa J, Kawamata S, Yamaoka K (2017) Development of arthrogenic joint contracture as a result of pathological changes in remobilized rat knees. J Orthop Res 35:1414–1423. https://doi.org/10.1002/jor.23419
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. https://doi.org/10.3758/BF03193146
McElroy JF, Wade GN (1987) Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav 39:361–365. https://doi.org/10.1016/0031-9384(87)90235-6
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–170. https://doi.org/10.1016/j.jsbmb.2013.09.008
Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA (1985) Skeletal alterations in ovariectomized rats. Calcif Tissue Int 37:324–328. https://doi.org/10.1007/BF02554882
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: A tutorial. Bone 14:595–608. https://doi.org/10.1016/8756-3282(93)90081-K
Wronski TJ, Cintrón M, Dann LM (1988) Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43:179–183. https://doi.org/10.1007/BF02571317
Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB (1987) Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol 122:49–60. https://doi.org/10.1016/0012-1606(87)90331-9
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int 34:285–290. https://doi.org/10.1007/BF02411252
Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12:17–25. https://doi.org/10.1016/j.molmed.2005.11.007
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238. https://doi.org/10.1002/jbmr.320
Jilka RL, Noble B, Weinstein RS (2013) Osteocyte apoptosis. Bone 54:264–271. https://doi.org/10.1016/j.bone.2012.11.038
Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS (1998) The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res 13:1243–1250. https://doi.org/10.1359/jbmr.1998.13.8.1243
Verborgt O, Tatton NA, Majeska RJ, Schaffler MB (2002) Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res 17:907–914. https://doi.org/10.1359/jbmr.2002.17.5.907
Delius M, Draenert K, Al Diek Y, Draenert Y (1995) Biological effects of shock waves: In vivo effect of high energy pulses on rabbit bone. Ultrasound Med Biol 21:1219–1225. https://doi.org/10.1016/0301-5629(95)00030-5
Sapir-Koren R, Livshits G (2014) Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption–formation cycles? Osteoporos Int 25:2685–2700. https://doi.org/10.1007/s00198-014-2808-0
Acknowledgements
We thank Mr. Masato Nomura, Mr. Yoshio Wakimoto, Mr. Ryota Suzuki, Mr. Takumi Yakuwa, Mr. Changxin Li, Mr. Taisei Wakigawa, Mr. Toshiya Tsubaki, and Ms. Sae Kinoshita for their skilled technical assistance; Asst. Prof. Akira Ito and Dr. Akihiro Nakahata for their support with mechanical testing; and Asst. Prof. Noriaki Maeshige for support with the ultrasound transducer. We are also grateful to Nihon Medix Co., Ltd. for providing the electrical stimulator; and SAKAI Medical Co., Ltd. for providing the radial extracorporeal shock wave device.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant No. 16K12933 and Suzuken Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shota Inoue, Junpei Hatakeyama, Hitoshi Aoki, Hiroshi Kuroki, Takahiro Niikura, Keisuke Oe, Tomoaki Fukui, Ryosuke Kuroda, Toshihiro Akisue, and Hideki Moriyama declare that they have no conflict of interest.
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee and performed according to the Kobe University Animal Experimentation Regulations (approval number: P160607).
Informed consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the incorrect version of supplementary material was corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inoue, S., Hatakeyama, J., Aoki, H. et al. Utilization of Mechanical Stress to Treat Osteoporosis: The Effects of Electrical Stimulation, Radial Extracorporeal Shock Wave, and Ultrasound on Experimental Osteoporosis in Ovariectomized Rats. Calcif Tissue Int 109, 215–229 (2021). https://doi.org/10.1007/s00223-021-00831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00831-6