Abstract
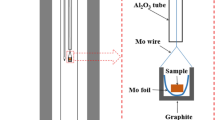
The solid-state reaction and diffusion behaviors of CaFe2O4 and TiO2 were investigated using a diffusion couple method at 1373 K to 1473 K under air atmosphere, and an in situ XRD method was used to analyze the solid-state reaction process. The reactions between calcium ferrite and TiO2 in the solid state are displacement reactions that produce perovskite and Fe2O3. The diffusion interface was divided into three layers based on phase analysis: layer I was composed of CaTiO3; layer II was composed of Fe2O3, CaTiO3, CaFe2O4, and Ca2Fe2O5; and layer III was composed of CaTiO3, Fe2O3, CaFe2O3, and CaFe4O7. The interface was formed in three stages. In stage I, CaTiO3 formed at the interface. In stage II, the gradual thickening of layers I and II was accompanied by the growth of the amount of CaTiO3 phase. In stage III, CF decomposed into CF2, and layer III formed. Micropores were formed in layer II due to the Kirkendall effect, and the diffusion rate of Ca2+ in CaFe2O4 was greater than that in CaTiO3. The squared thickness of layer I was found to depend linearly on time, and the expression for predicting the thickness of layer I was proposed:















Similar content being viewed by others
References
Geological Survey U. Mineral Commodity Summaries, 2020, pp. 176-77.
H. G. Du. Principle of Smelting Vanadium-titanium Magnetite in the Blast Furnace. Beijing: Science, 1996.
D. Fernández-González, I. Ruiz-Bustinza, J. Mochón, C. González-Gasca,L. F. Verdeja: Miner. process extr. m. 2017, vol. 38, pp. 215-27.
P. R. Dawson, J. Ostwald, K.M. Hayes: T. I. Min. Metall. C, 1985, vol. 94, pp. 71-8.
I. Shigaki, M. Sawada, N. Gennai: T. Iron Steel I. Japan, 1986, vol. 26, pp. 503-11.
C. E. Loo, K. T. Wan, V. R. Howes: Ironmaking Steelmaking, 1988, vol. 15, pp. 279-85.
Boyuan Cai, Takashi Watanabe, Chikashi Kamijo, Masahiro Susa, Miyuki Hayashi: ISIJ. Int., 2018, vol. 58, pp. 642-51.
Y. Hida, M. Sasaki, K. Sato, M. Kagawa, T. Miyazaki, H. Soma, H. Naito, M. Taniguchi: Nippon Steel Tech. Rep., 1987, vol. 35, pp. 59-67.
Y. Hida, J. Okazaki, K. Itoh, and M. Sasaki: Tetsu-to-Hagané, 1987, vol. 73, pp. 1893–1900.
M. Sasaki and Y. Hida: Tetsu-to-Hagané, 1982, vol. 68, pp. 563–71.
L. H. Hsieh, J.A. Whiteman: ISIJ Int., 1989, vol. 29, pp. 625-34.
N. V. Y. Scarlett, M. I. Pownceby, I. C. Madsen, A.N. Christensen: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 929-36.
N. V. Y. Scarlett, I. C. Madsen, M.I. Pownceby, A.N. Christensen: J. Appl. Crystallogr., 2004, vol. 37, pp. 362-68.
N. A. S. Webster, M. I. Pownceby, I. C. Madsen, J. A. Kimpton: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1344-57.
N. J. Bristow, C.E. Loo: ISIJ Int., 1992, vol. 32, pp. 819-28.
A. D. Manshadi, J. Manuel, S. Hapugoda, N. Ware: ISIJ Int., 2014, vol. 45, pp. 2189-95.
T. Pannanen, K. Kinnunen: Steel Res. Int., 2009, vol. 80, pp. 408-14.
H. P. Pimenta, V. Seshadri: Ironmak. Steelmak., 2002, vol. 29, pp. 175-79.
M. Zhou, S. T. Yang, T. Jiang, X.X. Xue: Ironmak. Steelmak., 2015, vol. 42, pp. 217-25.
W. S. Wang, G. Ren, Y. Q. Sun, Q. Lv: Iron Steel., 2010, vol. 45, pp. 13-17.
E. Park, O. Ostrovski: ISIJ Int., 2004, vol. 44, pp. 74-81.
M. Zhou, S. T. Yang, T. Jiang, and X.X. Xue: JOM, 2015, vol. 67, pp. 1203-13.
S. Ren, J. L. Zhang, X. D. Xing, B. X. Su, Z. Wang, B. J. Yan: Ironmak. Steelmak., 2014, vol. 41, pp. 500-06.
M. Suzuki, T. Tanaka: ISIJ Int., 2010, vol. 59, pp. 509-14.
C. Y. Ding, X. W. Lv, Y. Chen, G. Li, W. C. He. X. M. Lv: J. Alloy Compd., 2019, 789. pp. 537-46.
M. R. Yang, X. W. Lv, R. R. Wei, C. G. Bai. : Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2667-80.
H. Fukuyama, Md. Khalid Hossain, and K. Nagata: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 257–64.
K. Nagata, K. Sato, K. S. Goto.: Metall. Trans. B, 1980, vol. 11B, pp. 455-61.
L. Gao, L. Zhou, C. S. Li, J. Q. Feng, Y. F. Lu: J Mater Sci, 2013, vol. 48. pp. 974-977.
K. G. Shi, K .C. Zhou, L. Zhang, Z. Y. Li.: J. Cent. South Univ. 2012, vol. 19, pp. 2411-15.
K. T. Jacob, Sapna Gupta: Bull. Mater. Sci., 2009, vol. 32, pp. 611-16.
V. Daněk, I. Nerád: Chem. Pap. 2002, vol. 56, pp. 241-46.
R. M. Vezikova and V. M. Gropyanov: Refractories, 1993, vol. 34, pp. 87-90.
S. Forsberg, P. Wikström & E. Rosén: Metall. Trans. B, 2002, vol. 33B, pp. 385-92.
B. F. Decker, J. S. Kasper: Acta Cryst. 1957, vol. 10, pp. 332-34.
A. D. Smigelskas and E. O. Kirkendall: Trans. AIME, vol. 171, 1947, pp. 130.
A. Paul, T. Laurila, V. Vuorinen, and S.V. Divinski: Thermodynamics, Diffusion and the Kirkendall Effect in Solids, Springer, Cham.
John D. Whittenberger: Metall. Trans., 1972, vol. 3, 3038-40.
Isao Sakaguchi, Hajime Haneda: J. Solid State Chem. 1996, 124, pp. 195-97.
M. Ceh, D. Kolar: J. Mater. Sci., 1989, vol. 24, pp. 4307-10.
Patricia M. Hillm, H. S. Peiser, J. R. Pait. Acta Crystallogr. 1956, 9. pp. 981-86.
Roushown Ali, Masatomo Yashima. J. Solid State Chem. 2005, 178, 2867-72.
Acknowledgments
This work was supported by the Key Fund of Natural Science (No. 51974048), Chongqing Outstanding Youth Project (No. cstc2019jcyjjqX0024), Chongqing Postdoctoral Innovation Program (CQBX201904), as well as the Chongqing Key Laboratory of Vanadium-Titanium Metallurgy and New Materials.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted July 3, 2020; accepted February 6, 2021.
Rights and permissions
About this article
Cite this article
Yang, M., Xiang, J., Bai, C. et al. Solid-State Reaction and Diffusion Behaviors of CaFe2O4 and TiO2 at 1373 K to 1473 K. Metall Mater Trans B 52, 1436–1449 (2021). https://doi.org/10.1007/s11663-021-02111-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02111-y