Abstract
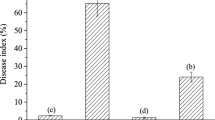
Plant antimicrobial peptides (AMPs) as a part of plant defense responses, are small soluble defense molecules which can inhibit the growth of pathogens. This study evaluates the effect of an antimicrobial peptide obtained from Allium sativum (AsR416) on Rhizoctonia solani (AG1-IA) the causal agent of rice sheath blight, in vitro and in vivo conditions. Firstly, the obtained results revealed that AsR416 (100 mg ml−1) inhibited the growth and sclerotia production of R. solani AG1-IA. Furthermore, these results showed the mode of action and mechanisms of AsR416 effect in inhibiting sclerotia formation of R. solani AG1-IA via metabolomics tools. AsR416 decreased the biomass of R. solani AG1-IA in liquid culture. In addition, nitro blue tetrazolium and evans blue staining methods revealed that the antimicrobial peptide induced O2− formation in the hyphal cells and mycelia cell death of R. solani AG1-IA, respectively. AsR416 delayed the pathogen infection process and decreased the severity of rice sheath blight disease in vitro and in vivo conditions. AsR416 reduced activity of cellulase, which is one of the virulence factors of this pathogen. The number of sclerotia decreased on plants treated with AsR416 after 2 months compared with the control. Considering the need to reduce application of hazardous synthetic fungicides against pathogenic fungi, using AMPs could be a successful method to increase rice production and reduce the use of chemical fungicides against sheath blight disease.





Similar content being viewed by others
References
Aarbiou, J., Tjabringa, G. S., Verhoosel, R. M., Ninaber, D. K., White, S. R., Peltenburg, L. T. C., & Hiemstra, P. S. (2006). Mechanisms of cell death induced by the neutrophil antimicrobial peptides a-defensins and LL-37. Inflammation Research, 55(3), 119–127.
Abd-El-Khair, H., & El-Gamal Nadia, G. (2011). Effect of aqueous extracts of some plant species against Fusarium solani and Rhizoctonia solani in Phaseolus vulgaris plants. Archives Phytopathology Plant Protection, 44, 1–16.
Aerts, A. M., Carmona-Gutierrez, D., Lefevre, S., Govaert, G., Francois, I. E., Madeo, F., Santos, R., Cammue, B. P., & Thevissen, K. (2009). The antifungal plant defensing RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Letters, 583(15), 2513–2516.
Aliferis, K. A., & Jabaji, S. (2010). 1H NMR and GC-MS metabolic fingerprinting of developmental stage of Rhizoctonia solani sclerotia. Metabolomics, 6, 96–108.
Andreu, D., & Rivas, L. (1998). Animal antimicrobial peptides: An overview. Biopolymers, 47, 415–433.
Baehner, R. L., Boxer, L. A., & Davis, J. (1976). The biochemical basis of nitroblue Tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood, 48(2), 303–313.
Broekaert, W. F., Cammue, B. P. A., De Bolle, M. F. C., Thevissen, K., De Samblanx, G. W., & Osborn, R. W. (1997). Antimicrobial peptides from plants. Critical Reviews in Plant Science, 16(3), 297–323.
Broekaert, W. F., Marien, W., Terras, F. R., De Bolle, M. F., Proost, P., Van Damme, J., Dillen, L., Claeys, M., Rees, S. B., Vanderleyden, J., & Cammue, B. P. A. (1992). Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry, 31, 4308–4314.
Bulet, P., Stocklin, R., & Menin, L. (2004). Antimicrobial peptides: From invertebrates to vertebrates. Immunological Reviews, 198, 169–184.
Cammue, B. P. A., Debolle, M. F. C., Terras, F. R. G., Proost, P., Vandamme, J., Rees, S. B., Vanderleyden, J., & Broekaert, W. F. (1992). Isolation and characterization of a novel class of plant antimicrobial peptides form Mirabilis Jalapa L. seeds. The Journal of Biological Chemistry, 267, 2228–2233.
Cammue, B. P. A., Thevissen, K., Hendriks, M., Eggermont, K., Goderis, I. J., Proost, P., Damme, J. V., Osborn, R. W., Guerbette, F., Kader, J. C., & Broekaerrt, W. F. (1995). A potent antimicrobial protein from onion seeds showing sequence homology to plant liquid transfer proteins. Plant Physiology, 109, 445–455.
Candido, E. S., Pinto, M. F., Pelegrini, P. B., Lima, T. B., Silva, O. N., Pogue, R., Grossi-Des-Sa, M. F., & Franco, O. L. (2011). Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB Journal, 25(10), 3290–3305.
Carling, D. E., Kuninaga, S., & Brainard, K. A. (2002). Hyphal anastomosis reactions, rDNA-internal transcribed spacer sequences, and virulence levels among subsets of Rhizoctonia solani anastomosis group-2 (AG-2) and AG-BI. Phytopathology, 92, 43–50.
Carpita, N. C., & Gibeaut, D. M. (1993). Structural models of primary cell walls in flowering plants, consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal, 3, 1–30.
Chet, I., Henis, Y., & Mitchell, R. (1966). The morphogenetic effect of Sulphur- containing amino acids, glutathione and iodoacetic acid on Sclerotium rolfsii. Journal of General Microbiology, 45, 541–546.
Cho, J., Hwang, I. S., Choi, H., Hwang, J. H., Hwang, J. S., & Lee, D. G. (2012). The novel biological action of antimicrobial peptides via apoptosis induction. Journal of Microbiology and Biotechnology, 22(11), 1457–1466.
Ciampi, M. B., Meyer, M. C., Costa, M. J. N., Zala, M., Macdonald, B. A., & Ceresini, P. C. (2008). Genetic structure of populations of Rhizoctonia solani anastomosis group-1 IA from soybean in Brazil. Phytopathology, 98(8), 932–941.
Cooke, R. C. (1969). Changes in soluble carbohydrates during sclerotium formation by Sclerotinia sclerotiorum and Sclerotinia trifoliorum. Transactions of the British Mycological Society, 58, 77–86.
Cooter, P. D., Hill, C., & Ross, P. (2005). Bacterial lantibiotics: Strategies to improve therapeutic potential. Current Protein and Peptide Science, 6, 61–75.
Craik, D. J., Clark, R. J., & Daly, N. L. (2007). Potential therapeutic applications of the cyclotides and related cysteine knot mini-proteins. Expert OpinInvestig Drugs, 16(5), 595–604.
Datta, A., Ghosh, A., Airoldi, C., Sperandeo, P., Mroue, K. H., Jimenez-Barbero, J., Kundu, P., Ramamoorthy, A., & Bhunia, A. (2015). Antimicrobial peptides: Insights into membrane Permeabilization, lipopolysaccharide fragmentation and application in plant disease control. Scientific Reports, 5, 11951.
De Lucca, A. J., Jacks, T. J., & Broekaert, W. J. (1998). Fungicidal and binding properties of three plant peptides. Mycopathologia, 144(2), 87–91.
Degenkolb, T., Berg, A., Gams, W., Schlegel, B., & Grafe, U. (2003). The occurrence of peptaibols and structurally related peptaibiotics in fungi and their mass spectrophotometric identification via diagnostic fragment ions. Journal of Peptide Science, 9, 666–678.
Ebrahim-Nesbat, F., Boh, S., Heitefuss, R., & Apel, K. (1993). Thionin in cell walls and papillae of barley in compatible and incompatible interactions with Erysiphe graminis f. sp. Hordei. Physiological and Molecular Plant Pathology, 43(5), 343–352.
Egorov, T. A., Odintsova, T. I., Pukhalsky, V. A., & Grishin, E. V. (2005). Diversity of wheat anti-microbial peptides. Peptides, 26(11), 2064–2073.
Ferre, R., Badosa, E., Feliu, L., Planas, M., Montesinos, E., & Bardai, E. (2006). Inhibition of plant-pathogenic bacteria by short synthetic cercropin A-melittin hybrid peptides. Applied and Environmental Microbiology, 72(5), 3302–3308.
Gao, G. H., Liu, W., Dai, J. X., Wang, J. F., Hu, Z., Zhang, Y., & Wang, D. C. (2001). Solution structure of PAFP-s: A new knottin-type antifungal peptide from the seeds of Phytolacca americana. Biocchemistry, 40(37), 10973–10978.
Ghose, T. K. (1987). Measurement of cellulose activities. Pure and Applied Chemistry, 59, 257–268.
Granade, T. C., Heeann, M. F., & Artis, W. M. (1985). Monitoring of filamentous fungal growth by in situ microspectrophotometry, fragmented mycelium absorbance density and 14C incorporation: Alternative to mycelial dry weight. Applied and Environmental Microbiology, 49(1), 101–108.
Groth, D. E. (2008). Effects of cultivar resistance and single fungicide application on rice sheath blight, yield and qual-ity. Crop Protection, 27, 1125–1130.
Hadacek, F., & Greger, H. (2000). Testing of antifungal natural products: Methodologies, comparability of results and assay choice. Phytochemical Analysis, 11, 137–174.
Hamlyn, P. F., Bradshaw, R. E., Mellon, F. M., Santiago, C. M., Wilson, J. M., & Peberdy, J. F. (1981). Efficient protoplast isolation from fungi using commercial enzymes. Enzyme and Microbial Technology, 3, 321–325.
Harholt, J., Suttangkakul, A., & Vibe Scheller, H. (2010). Biosynthesis of pectin. Plant Physiology, 153, 384–395.
Hausner, G., & Reid, J. (1999). Factors influencing the production of sclerotia in the wild rice (Zizania aquatica) pathogen Sclerotium hydrophilum. Mycoscience, 40, 393–400.
Hayes, B. M., Bleackley, M. R., Wiltshire, J. L., Anderson, M. A., Traven, A., & Van DerWeerden, N. L. (2013). Identification and mechanism of action of the plant defensing NaD1 as a new member of antifungal drug arsenal against Candida albicans. Antimicrobial Agents and Chemotherapy, 57, 3667–3675.
Henis, Y., Okon, Y., & Chet, I. (1973). The relationship between early hyphal branching and formation of sclerotia in Sclerotium rolfsii. Journal of General Microbiology, 79, 147–150.
Islam, W., Tayyab, M., Khalil, F., Hua, Z., Huang, Z., & Chen, H. Y. H. (2020). Silicon-mediated plant defense against pathogens and insect pests. Pesticide Biochemistry and Physiology, 168, 104641.
Jack, R. W., & Jung, G. (2000). Lantibiotics and microcins: Polypeptides with unusual chemical diversity. Current Opinion in Chemical Biology, 4, 310–317.
Jennings, C., West, J., Waine, C., Craik, D., & Anderson, M. (2001). Biosynthesis and insecticidal properties of plant cyclotides: The cyclic knotted proteins from Oldenlandia affinis. PNAS, 98(19), 10614–10619.
Kritzman, G., Okon, Y., Chet, I., & Henis, Y. (1976). Metabolism of L-threonine and its relationship to sclerotium formation in Sclerotium rolfsii. Journal of General Microbiology, 95, 78–86.
Lai, F. M., DeLong, C., Mei, K., Wignes, T., & Fobert, P. R. (2002). Analysis of the DRR230 family of pea defensins: Gene expression pattern and evidence of broad host-range antifungal activity. Plant Science, 163, 855–864.
Lay, F. T., & Anderson, M. A. (2005). Defensins- components of the innate immune system in plants. Current Protein and Peptide Science, 6, 85–101.
Lazzaro, B. P., Zasloff, M., & Rolff, J. (2020). Antimicrobial peptides: Application informed by evolution. Science, (6490), 368, eaau5480.
Liu, M. F., & Wu, L. C. (1971). The effect of amino acids on the growth and morphogenesis of Sclerotium rolfsii. Plant Protection Bulletin, Taiwan, 13, 87–96.
Lopez-Garcia, B., Segundo, B. S., Coca, M. (2012). Antimicrobial peptides as a promising alternative for plant disease protection. In: Peptides for disease control. American ChemicalSociety, Washington DC.
MacMillan, J. D., & Voughin, R. H. (1964). Purification and properties of polyglacturonic acid- transeliminase produced by Clastridium multiformentans. Biochemistry, 3, 564–572.
Madeo, F., Frohlich, E., & Frohlich, K. U. (1997). A yeast mutant showing diagnostic markers of early and late apoptosis. The Journal of Cell Biology, 139(3), 729–734.
Maly, F. F., Nakamura, M., Gauchat, J. F., Urwyler, A., Walker, C., Dahinden, C. A., Cross, A. R., Jones, O. T., & de Weck, A. L. (1989). Superoxide- dependent nitroblue tetrazolium reduction and expression of cytochrome b- 245 component by human tonsillar B lymphocytes and B cell line. The Journal of Immunology, 142(4), 1260–1267.
McManus, A. M., Nielsen, K. J., Marcus, J. P., Harrison, S. J., Green, J. L., Manners, J. M., & Craik, D. J. (1999). MiAMP1, a novel protein from Macadamia integrifolia adopts a greek key beta-barrel fold unique amongst plant antimicrobial protein. Journal of Molecular Biology, 293, 629–638.
Meena, M., Prasad, V., Zehra, A., Gupta, V. K., & Upadhyay, R. S. (2015). Mannitol metabolism during pathogenic fungal-host interactions under stressed conditions. Frontiers in Microbiology, 6, 1–12.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Money, N. P. (2016). Fungal cell biology and development. In: The fungi. Elsevier 37–66.
Montesinos, E. (2007). Antimicrobial peptides and plant disease control. FEMS Microbial Letter, 270, 1–11.
Moromizato, Z., Ishizaki, F., Takara, K., & Tamori, M. (1991). The effect of phosphorus and magnesium on sclerotium formationin Rhizoctonia solani Kuhn. Annual phytopathology Society of Japan, 57, 649–656.
Olutiola, P. O., & Cole, O. O. (1976). Production of a cellulose complex in culture filtrates of Aspergillus tamari associated with mouldy cocoa beans in Nigeria. Physiologia Plantarum, 37, 313–316.
Peters, B. M., Shirtliff, M. E., & Jabra-Rizk, M. A. (2010). Antimicrobial peptides: Primeval molecules or future drug? PLoS Pathogens, 6(10), e1001067.
Segura, A., Moreno, M., Madueno, F., Molina, A., & Garcia-Olmedo, F. (1999). Snakin-1, a peptide from potato that is active against plant pathogen. Molecular Plant Microbe Interaction, 12(1), 16–23.
Semighini, C. P., Harris, S. D. (2010). Methods to detect apoptotic-like cell death in filamentous fungi. IN: Molecular and cell biology methods for fungi, methods in molecular biology. Springer science + Business Media. 317 pp.
Stintzi, A., Heitz, T., Prasad, V., Wiedemann-Merdinoglu, S., Kauffmann, S., Geoffroy, P., Legrand, M., & Fritig, B. (1993). Plant "pathogenesis-related" proteins and their role in defense against pathogens. Biochimie, 75, 687–706.
Swidergall, M., & Ernst, J. F. (2014). Interplay between Candida albicans and antimicrobial peptide armory. Eukaryotic Cell, 13(8), 950–957.
Taheri, P. (2018). Cereal diseases caused by Fusarium graminearum: From biology of the pathogen to oxidative burst-related host defense responses. European Journal of Plant Pathology, 152(1), 1–20.
Taheri, P., Gnanamanickam, S., & Hofte, M. (2007). Characterization, genetic structure, and pathogenicity of Rhizoctonia spp. associated with rice sheath disease in India. Phytopathology, 97(3), 373–383.
Taheri, P., & Tarighi, S. (2010). Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. Journal of Plant Physiology, 167, 201–208.
Taheri, P., & Tarighi, S. (2011). Cytomolecular aspects of rice sheath blight caused by Rhizoctonia solani. European Journal of Plant Pathology, 129, 511–528.
Tailor, R. H., Acland, D. P., Attenborough, S., Cammue, B. P., Evans, I. J., Osborn, R. W., Ray, J. A., Rees, S. B., & Broekaert, W. F. (1997). A novel family of small cysteine-rich antimicrobial peptides from seed of Impatiens blasamina is derived from a single precursor protein. The Journal of Biological Chemistry, 272(39), 24480–24487.
Tam, J. P., Wang, S., Wong, K. H., & Tan, W. L. (2015). Antimicrobial peptides from plants. Pharmaceutical, 8, 711–757.
Terras, F. R., Eggermont, K., Kovaleva, V., Raikhel, N. V., Osborn, R. W., Kester, A., Rees, S. B., Torrekens, S., Leuven, F. V., Vanderleyden, J., Cammue, B. P. A., & Broekaert, W. F. (1995). Small cysteine-rich antifungal proteins from radish: Their role in host defense. The Plant Cell, 7, 573–588.
Terras, F. R., Schoofs, H. M., DeBolle, M. F., Van Leuven, F., Rees, S. B., Vanderleyden, J., Cammue, B. P., & Broekaert, W. F. (1992). Analysis of two novel classes of plant antifungal proteins from radish seeds. The Journal of Biological Chemistry, 5(267), 15301–15309.
Thaler, J. S., Fidantsef, A. L., Duffey, S. S., & Bostock, R. M. (1993). Trade-offs in plant defense against pathogens and herbivores: A field demonstration of chemical elicitors of induced resistance. Journal of Chemical Ecology, 25, 1597–1609.
Tincu, J. A., & Taylor, S. W. (2004). Antimicrobial peptides from marine invertebrates. Antimicrobial Agents and Chemotherapy, 48, 3645–3654.
Topman, S., Tamir-Ariel, D., Bochnic-Tamir, H., Bauer, T. S., Shafir, S., Burdman, S., & Hayouka, Z. (2018). Random peptide mixtures as new crop protection agents. Microbial Biotechnology, 11(6), 1027–1036.
Vila-Perello, M., Sanchez-Vallet, A., Garcia-Olmedo, F., Molina, A., & Andreu, D. (2003). Synthetic and structural studies on Pyrularia pubera thionin: A single-residue mutation enhance activity against gram- negative bacteria. FEBS Letters, 536, 215–219.
Xi, K., Yang, M., Abbas, H. M. K., Wu, J., Li, M., & Dong, W. (2018). Antimicrobial genes from Allium sativum and Pinellia ternate revealed by Bacillus subtilis expression system. Scientific Reports, 8, 14514.
Yount, N. Y., & Yeaman, M. R. (2013). Peptide antimicrobials: Cell wall as a bacterial target. Annals of the New York Academy of science, 1277, 127–138.
Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms. Nature, 415, 389–395.
Zhang, L., Rozek, A., Robert, E., & Hancock, R. E. (2001). Interaction of cationic antimicrobial peptides with model membranes. The Journal of Biological Chemistry, 276, 35714–35722.
Zheng, A., Lin, R., Zhang, D., Qin, P., Xu, L., Ai, P., Ding, L., Wang, Y., & Chen, Y. (2013). The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nature Communications, 4(1424), 1–10.
Acknowledgments
We thank Ferdowsi University of Mashhad, Iran, for financial support of this research with project number 3/43459 approved on 3 April 2017. Also, this research was partly supported by Huazhong University, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This manuscript complies to the ethical rules applicable for this journal.
Rights and permissions
About this article
Cite this article
Nassimi, Z., Taheri, P., Kong, X. et al. The antimicrobial peptide AsR416 can inhibit the growth, sclerotium formation and virulence of Rhizoctonia solani AG1-IA. Eur J Plant Pathol 160, 469–485 (2021). https://doi.org/10.1007/s10658-021-02257-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02257-0