Abstract
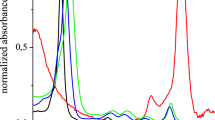
Antibacterial photodynamic therapy (APDT) is a promising method of treatment of local infections, based on photochemical reaction of photosensitizer (PS) under irradiation with visible or near infra-red light of appropriate wavelength. A molecule of PS is activated by light energy and as a result singlet oxygen and other reactive oxygen species, which are toxic for microbes, are generated. Antibacterial action of new PS (synthetic polycationic bacteriochlorin derivatives with spectral maximum of absorbance at 760 nm) with improved features was investigated in vitro in this work. Tetra- and octacationic bacteriochlorins with lower molecular mass, as compared with ones previously proposed, turned out to be highly effective against Pseudomonas aeruginosa and Staphylococcus aureus in plankton and biofilm state: reduction of viable bacteria in biofilms reached 5log10. The dependence of the effect on PS concentration, the time of incubation with PS and light dose was ascertained. Light microscopy of biofilms after photodynamic treatment and Live/Dead staining confirmed the death of bacteria or damage of their membranes after exposure to PS and irradiation.






Similar content being viewed by others
REFERENCES
Wainwright, M., Maisch, T., Nonell, S., Plaetzer, K., Almeida, A., Tegos, G.P., et al., Photoantimicrobials-are we afraid of the light?, Lancet Infect. Dis., 2017, vol. 17, no. 2, pp. e49–e55. https://doi.org/10.1016/S1473-3099(16)30268-7
Tavares, A., Carvalho, C.M., Faustino, M.A., Neves, M.G., Tomé, J.P., Tomé, A.C., et al., Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment, Mar. Drugs, 2010, vol. 8, no. 1, pp. 91–105. https://doi.org/10.3390/md8010091
Vera, D.M., Haynes, M.H., Ball, A.R., Dai, T., Astrakas, C., Kelso, M.J., et al., Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes, Photochem. Photobiol., 2012, vol. 88, no. 3, pp. 499–511.https://doi.org/10.1111/j.1751-1097.2012.01087.x
Tseng, S.P., Teng, L.J., Chen, C.T., Lo, T.H., Hung, W.C., Chen, H.J., et al., Toluidine blue O photodynamic inactivation on multidrug resistant Pseudomonas aeruginosa, Lasers Surg. Med., 2009, vol. 41, no. 5, pp. 391–397. https://doi.org/10.1002/lsm.20765
Beirão, S., Fernandes, S., Coelho, J., Faustino, M.A., Tomé, J.P., Neves, M.G., et al., Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin, Photochem. Photobiol., 2014, vol. 90, no. 6, pp. 1387–1396. https://doi.org/10.1111/php.123317
Simpson, J.A., Smith, S.E., and Dean, R.T., Scavenging by alginate of free radicals released by macrophages, Free Radical Biol. Med., 1989, vol. 6, no. 4, pp. 347–353. https://doi.org/10.1016/0891-5849(89)90078-6
Borriello, G., Werner, E., Roe, F., et al., Oxygen limitation contributes to antimicrobial tolerance of Pseudomonas aeruginosa in biofilms, Antimicrob. Agents Chemother., 2004, vol. 48, pp. 2659–2664. https://doi.org/10.1128/AAC.48.7.2659-2664.2004
Bjarnsholt, Th., Jensen, P.O., Moser, C., and Hoiby, N., in Biofilm Infections, Bjarnsholt, Th., Jensen, P.O., Moser, C., and Hoiby, N., Eds., Springer Science + Business Media, 2011. https://doi.org/10.1007/978-1-4419-6084-9
Almeida, A., Cunha, A., Faustino, M.A.F., Tome, A.C., and Neves, M.G.P.M.S., Porphyrins as antimicrobial photosensitizing agents, in Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications, Hamblin, M.R. and Jori, G., Eds., Cambridge: Royal Society of Chemistry, 2011. https://doi.org/10.1039/9781849733083-00083
George, S., Hamblin, M.R., and Kishen, A., Uptake pathways of anionic and cationic photosensitizers into bacteria, Photochem. Photobiol. Sci., 2009, vol. 8, no. 6, pp. 788–795. https://doi.org/10.1039/B809624D
Strakhovskaya, M.G., Antonenko, Y.N., Pashkovskaya, A.A., Kotova, E.A., Kireev, V., Zhukhovitsky, V.G., et al., Electrostatic binding of substituted metal phthalocyanines to enterobacteria cells: its role in photodynamic inactivation, Biochemistry (Moscow), 2009, vol. 74, no. 12, pp. 1305–1314. https://doi.org/10.1134/S0006297909120025
de Melo, W.C., Avci, P., de Oliveira, M.N., Gupta, A., Vecchio, D., Sadasivam, M., et al., Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection, Expert Rev. Anti-Infect. Ther., 2013, vol. 11, no. 7, pp. 669–693. https://doi.org/10.1586/14787210.2013.811861
Dai, T., Tegos, G.P., Lu, Z., Huang, L., Zhiyentayev, T., Franklin, M.J., et al., Photodynamic therapy for Acinetobacter baumannii burn infections in mice, Antimicrob. Agents Chemother., 2009, vol. 53, no. 9, pp. 3929–3934. https://doi.org/10.1128/AAC.00027-09
Yin, R., Agrawal, T., Khan, U., Gupta, G.K., Rai, V., Huang, Y.Y., et al., Antimicrobial photodynamic inactivation in nanomedicine: small light strides against bad bugs, Nanomedicine (London, U. K.), 2015, vol. 10, no. 15, pp. 2379–2404. https://doi.org/10.2217/nnm.15.67
Meerovich, G.A., Akhlyustina, E.V., Tiganova, I.G., Lukyanets, E.A., Makarova, E.A., Tolordava, E.R., et al., Novel polycationic photosensitizers for antibacterial photodynamic therapy, in Advances in Experimental Medicine and Biology, vol. 1282: Advances in Microbiology, Infectious Diseases and Public Health, Donelli, J., Ed., Cham: Springer, 2019. https://doi.org/10.1007/5584_2019_431
Douplik, A., Saiko, G., Schelkanova, I., and Tuchin, V., The response of tissue to laser light, in Lasers for Medical Applications—Diagnostics, Therapy and Surgery, Woodhead Publishing Series in Electronic and Optical Materials, Woodhead Publ., 2013, vol. 3, pp. 47–109. https://doi.org/10.1533/9780857097545.1.47
Meerovich, I., Nichols, M.G., and Dash, A.K., Low-intensity light-induced paclitaxel release from lipid-based nano-delivery systems, J. Drug Targeting, 2019, vol. 27, no. 9, pp. 971–983. https://doi.org/10.1080/1061186X.2019.1571066
Dudkin, S.V., Makarova, E.A., Slivka, L.K., and Lukyanets, E.A., Synthesis and properties of tetra- and octacationic meso-tetrakis(3-pyridyl)bacteriochlorin derivatives, J. Porphyrins Phthalocyanines, 2014, vol. 18, no. 01n02, pp. 107–114. https://doi.org/10.1142/s1088424613501162.
Tiganova, I.G., Makarova, E.A., Meerovich, G.A., Alekseeva, N.V., Tolordava, E.R., Zhizhimova, Y.S., et al., Photodynamic inactivation of pathogenic bacteria in biofilms using new synthetic bacteriochlorin derivatives, Biomed. Photonics, 2017, vol. 6, no. 4, pp. 27–36. https://doi.org/10.24931/2413-9432-2017-6-4-27-36
Makarova, E.A., Meerovich, G.A., Lukyanets, E.A., Tiganova, I.G., Romanova, Yu.M., Loschenov, V.B., et al., RF Patent 2670201, 2018. http://www.freepatent.ru/patents/2670201.
Huang, L., Huang, Y.Y., Mroz, P., Tegos, G.P., Zhiyentayev, T., Sharma, S.K., et al., Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers, Antimicrob. Agents Chemother., 2010, vol. 54, no. 9, pp. 3834–3841. https://doi.org/10.1128/AAC.00125-10
Wainwright, M., Photodynamic antimicrobial chemotherapy (PACT), J. Antimicrob. Chemother., 1998, vol. 42, pp. 13–28. https://doi.org/10.1093/jac/42.1.13
Friedrich, C.L., Moyles, D., Beveridge, T.J., et al., Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria, Antimicrob. Agents Chemother., 2000, vol. 44, pp. 2086–2092. https://doi.org/10.1128/aac.44.8.2086-2092.2000
George, S., Hamblin, M.R., and Kishena, A., Uptake pathways of anionic and cationic photosensitizers into bacteria, Photochem. Photobiol. Sci., 2009, vol. 8, no. 6, pp. 788–795. https://doi.org/10.1039/b809624d
Orekhov, Ph.S., Kholina, E.G., Bozdaganyan, M.E., Nesterenko, A.M., Kovalenko, I.B., and Strakhovskaya, M.G., Molecular mechanism of uptake of cationic photoantimicrobial phthalocyanine across bacterial membranes revealed by molecular dynamics simulations, J. Phys. Chem. B, 2018, vol. 122, no. 14, pp. 3711−3722. https://doi.org/10.1021/acs.jpcb.7b11707
Awad, M.M., Tovmasyan, A., Craik, J.D., Batinic-Haberle, I., and Benov, L.T., Important cellular targets for antimicrobial photodynamic therapy, Appl. Microb. Cell Physiol., 2016, vol. 104, no. 8, pp. 7679–7688. https://doi.org/10.1007/s00253-016-7632-3
Simões, C., Gomes, M.C., Neves, M.G.P.M.S., Cunha, Â., Tomé, J.P.C., Tomé, A.C., et al., Photodynamic inactivation of Escherichia coli with cationic meso-tetraarylporphyrins-The charge number and charge distribution effects, Catal. Today, 2016, vol. 266, pp. 197–204. https://doi.org/10.1016/j.cattod.2015.07.031
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
ADDITIONAL INFORMATION
Meerovich G.A.—https://orcid.org/0000-0002-2046-5492
Romanova Yu. M.—https://orcid.org/0000-0002-8547-1711
Lukyanets E. A.—https://orcid.org/0000-0002-8853-6912
Gintsburg A. L.—https://orcid.org/0000-0002-6182-3866
Zhizhimova Yu.S.—https://orcid.org/0000-0002-0665-9821
Makarova E.A.—https://orcid.org/0000-0003-4144-6159
Alekseeva N.V.—https://orcid.org/0000-0001-8051-7170
Philipova N.I.—https://orcid.org/0000-0002-1368-9113
Tolordava E.R.—https://orcid.org/0000-0002-9920-2432
Tiganova I.G.—https://orcid.org/0000-0003-2340-2726
About this article
Cite this article
Tiganova, I.G., Zhizhimova, Y.S., Philipova, N.I. et al. Antibacterial Properties of Synthetic Cationic Bacteriochlorin Derivatives as Photosensitizers. Mol. Genet. Microbiol. Virol. 35, 248–256 (2020). https://doi.org/10.3103/S0891416820040096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416820040096