Abstract
Chalcopyrite (CuFeS2) is the most refractory copper mineral when treated in conventional sulfate media leaching systems. This is the first study to examine the use of a dimethyl sulfoxide (CH3SOCH3, DMSO) solution containing copper chloride (CuCl2) for the leaching of chalcopyrite. Leaching experiments for a copper concentrate composed mainly of chalcopyrite were conducted at ambient pressure at 313–413 K. The leaching fractions of Cu, Fe, S, Au, and As were investigated. It was found that 90% of the Cu was extracted in 2 h, and 94% was extracted in 4 h at 373 K, which is competitive with other conventional processes. A DMSO solution containing CuCl2 could selectively dissolve the valuable metals Cu and Au from chalcopyrite, but leave minerals with little economic value such as pyrite (FeS2) and As in the residue. Chalcopyrite is oxidized by cupric ion in proportion to the stoichiometric ratio of Cu in the concentrate to the initial cupric ion in the DMSO solution, which is enhanced by the presence of oxygen below 373 K.
Similar content being viewed by others
1 Introduction
Chalcopyrite (CuFeS2), as one of the most copper-abundant minerals, is an important copper resource. However, chalcopyrite is the most refractory copper mineral when treated using conventional sulfate media leaching systems. The main reason for the slow rate of chalcopyrite dissolution is the formation of a layer on the surface of the mineral that hinders dissolution, termed “passivation” [1]. The slow dissolution of chalcopyrite at ambient pressure in sulfate media has hindered its industrial application. Nevertheless, due to the continuous depletion of high-grade ores, it is expected that future hydrometallurgical alternatives for chalcopyrite treatment will increase in importance and application [2]. Dreisinger reviewed hydrometallurgical options, including Activox, Albion, Anglo American-University of British Columbia, Bactech, BIOCOP™, CESL copper, Dynatec, and total pressure oxidation processes, to prevent the passivation of chalcopyrite, and compared the advantages and disadvantages of the selected process options [3]. Li et al. reviewed the structure, fundamental mechanisms, and kinetics of chalcopyrite leaching [4].
Although extensive research on chalcopyrite leaching has been conducted, most studies have focused on using aqueous solutions, and few studies have investigated the use of organic solvents for the leaching of chalcopyrite. Solis-Marcíal and Lapidus investigated the use of polar organic solvents in combination with sulfuric acid (H2SO4) media for chalcopyrite leaching [5, 6]. They found that the use of acetone (CH3COCH3), ethylene glycol (CH2OHCH2OH), 2-propanol (CH3CH(OH)CH3), or methanol (CH3OH) together with sulfuric acid solutions favors the oxidative leaching of chalcopyrite, which results in more copper extraction in a relatively short time under ambient conditions. Ruiz-Sánchez and Lapidus further investigated the role of ethylene glycol in leaching chalcopyrite concentrates with hydrogen peroxide (H2O2) in aqueous sulfuric acid solutions under ambient conditions [7]. They concluded that ethylene glycol inhibits H2O2 decomposition catalyzed by the copper and iron ions present in the solutions, leading to an effective dissolution of chalcopyrite at room temperature.
Water exists abundantly in nature and has many excellent solvent properties. If water is appropriate for a given purpose, it should be used [8]. The use of nonaqueous solvents instead of water may increase fire, health, and environmental toxicity risks, leading to complicated processes and high risk management costs. However, it is of interest to employ nonaqueous solvents if they work more efficiently for a certain purpose. Dimethyl sulfoxide (CH3SOCH3, DMSO) is an aprotic polar solvent that is widely used in chemical industries. DMSO has a number of advantages, including the ability to dissolve many inorganic salts, low toxicity, low vapor pressure at ambient temperature, and resistance to oxidation and reduction. The authors found that a DMSO solution containing copper halide can dissolve precious metals more quickly than other leaching methods under mild conditions. Cu2+ in DMSO acts as a strong oxidant that dissolves many metal elements [9].
In this work, the use of a DMSO solution containing copper chloride was investigated for leaching a copper concentrate composed mainly of chalcopyrite. Leaching experiments were conducted to determine the copper dissolution fractions under various conditions. Chalcopyrite is also frequently associated with valuable minerals, such as precious metals, as well as minerals with little economic value, such as pyrite (FeS2). This necessitates costly separation processes such as leaching and flotation. Moreover, chalcopyrite may also contain “penalty” elements, such as As [10]. Therefore, the dissolution of Au and As in the copper concentrate was also investigated.
2 Materials and Methods
2.1 Materials
Indonesian copper concentrate, which is composed primarily of chalcopyrite, was used in this study. Table 1 shows the core chemical composition of the copper concentrate, which was analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES, Varian VISTA-MPX). The Cu content was 24.7 wt.%; therefore, the approximate composition of chalcopyrite is 70 wt.%. This concentrate contained 0.007 wt.% Au and 0.018 wt.% As. The XRD spectrum (Fig. S1) shows that the copper concentrate contains chalcopyrite, pyrite, and silica (SiO2). The copper concentrate was dried for 24 h at temperatures above 373 K prior to use in the experiments.
The solution used in this experiment was prepared using reagent-grade DMSO, anhydrous cupric chloride (CuCl2), and sodium chloride (NaCl), all of which were purchased from FUJIFILM Wako Pure Chemicals. Sulfur (first-grade reagent) was purchased from Junsei Chemical Co., Ltd.
2.2 Leaching of Chalcopyrite
Leaching experiments were conducted according to the following procedure, unless otherwise noted. First, 10 g of concentrate were added to a 1 L eggplant flask for the rotary evaporator, and then the DMSO solution containing copper chloride was added. The solution was prepared by dissolving two equivalents of CuCl2 and NaCl (78.7 mmol) in 200 mL of DMSO. The stoichiometric ratio of Cu in the concentrate to the initial cupric ion in the DMSO solution was 1:2. Adding NaCl ensures that CuCl2 dissolves and forms Cu(DMSO)3Cl+, Cu(DMSO)Cl3−, or CuCl42− in DMSO [11]. Therefore, NaCl enhances the dissolution of metals in DMSO solutions containing copper halides [9].
The evaporator was kept at ambient pressure, and the oil bath temperature was set to 373 K. The eggplant flask was then immersed in an oil bath and stirred for 4 h at 140 rpm. After leaching, the ore residue in the solution was immediately removed by vacuum filtration to terminate the reactions. The obtained residue was dried in a dryer heated to 383 K for 24 h, and the leachate was gradually cooled at 293–298 K for 24 h. A yellow precipitate was formed in the leachate (Fig. S2), collected by filtration under reduced pressure, washed using DMSO, and dried at 383 K for 24 h. The obtained precipitate was measured and analyzed using a scanning electron microscope energy dispersive X-ray spectrometer, SEM-EDS (JEOL JSM-6510A). The results confirmed that the precipitate was sulfur.
The ore residue was placed in aqua regis, a mixture of concentrated nitric acid and hydrochloric acid in a molar ratio of 1:3 and kept at 433 K for 4 h to dissolve all the contained Cu, Fe, S, Au, and As. Then, the Cu, Fe, S, Au, and As contents were quantitatively analyzed by ICP-OES. The extracted fraction for each element was calculated using the following equation:
where M is each element, that is, Cu, Fe, S, Au, or As, Wore is the weight of ore (g), CM0 is the concentration of M in the ore (wt.%), Wresidue is the weight of residue (g), and CM1 is the concentration of M in the residue (wt.%).
In addition, the principal components of the obtained residue and their morphology were analyzed and estimated using X-ray diffraction, (XRD, Bruker AXS D8ADVANCE), and SEM-EDS.
2.3 Time and Temperature Dependence of Leaching of Chalcopyrite
To investigate the effect of time on the leaching fraction of copper concentrate, leaching experiments were conducted for 0.5, 1.0, 2.0, 4.0, and 8.0 h. The temperature was set to 373 K. The leaching rate was calculated using the chemical composition of the residue analyzed by ICP-OES.
To investigate the effect of temperature on the leaching fraction of the copper concentrate, leaching experiments were conducted at temperatures of 313, 333, 353, 373, 393, and 413 K. The leaching time was fixed at 4 h. The leaching rate was calculated using the chemical composition of the residue analyzed by ICP-OES.
3 Results and Discussions
3.1 Leaching of Chalcopyrite Using DMSO Solution Containing Copper Chloride
Table 2 summarizes the results of leaching of the copper concentrate for 4 h at 373 K.
The extracted fraction of Cu from the copper concentrate was determined to be 94.1%, as 36.6 mmol of Cu was extracted from the 38.9 mmol present in the copper concentrate. The extracted fraction of Fe was determined to be 46.3%, as 20.4 mmol of Fe was extracted from 44.1 mmol present in the concentrate.
As for S, 47.8 mmol of S was extracted from the 86.8 mmol in the concentrate. The amount of S precipitate in the leachate was 41.1 mmol. As a preliminary experiment, the solubility of S in DMSO was measured to be approximately 20 g (625 mmol)/L-DMSO at 373 K. The solubility of S in DMSO at ambient temperature (298 K) is 0.126 g (3.94 mmol)/L [12]. Therefore, 47.0 mmol of the dissolved S in the leachate would have been expected to precipitate with the decrease in temperature. However, 6.68 mmol of S remained in the leachate even if the recovered precipitate was removed. This is because the generated sulfur crystals were too small to be completely recovered by filtration.
The extracted fraction of Au was determined to be 75% as 3 μmol of Au were extracted from 4 μmol of Au in the concentrate. ICP-OES analysis did not detect Au in the leachate because the concentration of Au was lower than the lower detection limit for liquids, that is, 50 ppm. The calculated Au concentration in the leachate was 2 ppm on a mass basis, which is lower than the lower detection limit of ICP-OES. Although Au was not confirmed to be present in the leachate, it is expected that the Au contained in the Cu concentrate could be extracted into the leachate.
Examination of the residue showed that 20 μmol of As out of the initial 24 μmol of As remained. The calculated As concentration in the leachate was 1 ppm on a mass basis. Therefore, ICP-OES analysis did not detect As in the leachate. These results suggest that most of the As did not dissolve in the DMSO solution containing CuCl2 and remained in the residue. This suggests that As could be separated from other valuable metals present in the concentrate.
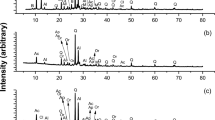
XRD analysis was performed to identify the components of the residue after leaching, and the results are shown in Fig. 1. According to the XRD spectrum, the main component of the residue is pyrite (FeS2). An optical microscope image of the residue is shown in Fig. 2, which indicates the presence of pyrite crystals. The residue was further analyzed by SEM-EDS, and the mapping results for each element are shown in Fig. 3. It can be seen that the surface of the residue is a compound of Fe and S. Because the concentrate contains pyrite in addition to chalcopyrite, this suggests that chalcopyrite dissolves preferentially over pyrite when leaching using a DMSO solution containing CuCl2.
From the stoichiometric results shown in Table 2, the contents of Fe and S in the residue are 23.7 and 39.1 mmol, respectively. The Fe/S ratio is 0.59, which is larger than the value of 0.5 for pure pyrite (FeS2). The contents of Cu and Fe in the leachate were 36.6 and 20.4 mmol, respectively. The Cu/Fe ratio is 1.8, which is much larger than the value of 1.0 for pure chalcopyrite (CuFeS2). Solis-Marcíal and Lapidus investigated the reaction mechanism for chalcopyrite leaching when methanol was used in combination with sulfuric acid media and CuSO4 and O3 as oxidants at 313 K [6]. Their results showed that iron dissolved in a lesser proportion relative to the copper extraction throughout the leaching process. They suggested that the chalcopyritic iron remained in the residue as FeS. Regarding iron extraction, the same behavior was observed in this study.
The mechanism of leaching the concentrate will be further discussed in the next section.
3.2 Time and Temperature Dependence of Chalcopyrite Leaching
The extracted fraction of Cu versus time is presented in Fig. 4, where the results obtained by previous studies are also shown for comparison. It was found that 90% of the Cu was extracted in 2 h, and 94% was extracted in 4 h at 373 K. Table 3 shows a detailed comparison of the leaching processes for chalcopyrite at ambient pressure. Although the system using DMSO solution is still in a fundamental research stage, the results for extracted Cu and Fe within a given length of time are competitive with other conventional processes. One of the advantages of using DMSO as a solvent is its high boiling point, which allows a high operating temperature at ambient pressure, leading to a high dissolution rate.
The extracted fraction of Cu in 4 h at various temperatures is shown in Fig. 5. The extracted fraction of Cu increased with an increase in temperature, reaching 94% at 373 K, but decreased with an increase in temperature from 373 to 413 K.
The extracted fraction of Fe in 4 h at a range of temperatures is shown in Fig. 6. The extracted fraction of Fe increased with an increase in temperature up to 373 K. However, unlike Cu, the extracted fraction of Fe did not decrease with an increase in temperature between 373 K and 413 K.
The main reaction for chalcopyrite dissolution in ferric/ferrous sulfate (aqueous) systems consists of the following anodic and cathodic half-cell reactions [2]:
Therefore, the reaction for chalcopyrite dissolution in cupric/cuprous DMSO systems may consist of the following anodic and cathodic half-cell reactions:
Then, the overall reaction is:
If oxygen exists in DMSO solutions, the following anodic and cathodic half-cell reactions take place [17, 18]:
Then, the overall reaction is:
The cupric ion is regenerated by this reaction and acts as an oxidant, as shown in Eq. (6). The ferrous ion may also be oxidized to a ferric ion and act as an oxidant. Therefore, the dissolution of chalcopyrite is enhanced by the presence of oxygen in the DMSO solution. An increase in the leaching temperature enhanced the dissolution of chalcopyrite (Eq. (6)) but decreased the dissolution of oxygen in the DMSO solution, inhibiting the dissolution of chalcopyrite. These are likely the reasons for the drop in the extracted fraction of copper above 373 K (Fig. 5).
Leaching experiments were conducted for different concentrations of CuCl2 (40 mmol and 118 mmol) with equivalent NaCl in DMSO for 4 h at 373 K and 413 K. The stoichiometric ratio of Cu in the concentrate to the initial cupric ion in the DMSO solution was 1:1 and 1:3, respectively. The results are shown in Fig. 7. It was observed that the dissolution of chalcopyrite was enhanced with an increase in the concentration of CuCl2 in the DMSO solution. When the concentration of CuCl2 in the DMSO solution decreased from 79 to 40 mmol, by approximately 50%, the extracted fraction of Cu at 373 K decreased from 94% to 63%, which was larger than the expected value of 47% based on the stoichiometric decrease in CuCl2 in the DMSO solution. When the concentration of CuCl2 in the DMSO solution increased from 79 to 118 mmol, the extracted fraction of Cu at 413 K increased proportionally from 63% to 99%. This result indicates that the leaching of chalcopyrite only takes place based on Eq. (6) at 413 K because of the low dissolution of oxygen in the DMSO solution.
In addition, preliminary leaching experiments for pyrite using the DMSO solution for 4 h at 373 K and 413 K were conducted. No dissolution of pyrite was observed at 373 K, whereas a small fraction of pyrite was dissolved at 413 K (Fig. S3). The increase in the extracted fraction of Fe with an increase in temperature from 393 K to 413 K is likely attributable to the dissolution of pyrite in the copper concentrate. The mechanism of dissolution of copper concentrate should be investigated further.
The recovery of Cu and Au from the leachate should also be investigated in future work. Previous work has shown that the precipitation of dissolved Au and Cu in DMSO solutions can be achieved by the addition of water [9]. Au and Cu from the leachate can be selectively precipitated by controlling the pH of the water. Electrowinning of Au and Cu from the leachate is also one possible method for recovery [19]. These methods should be integrated and optimized.
4 Conclusion
This study showed that a DMSO solution containing CuCl2 could dissolve chalcopyrite under mild conditions, while not dissolving pyrite. In addition, a DMSO solution containing CuCl2 dissolves precious metals, such as Au, but does not dissolve As contained in chalcopyrite ore. Therefore, a DMSO solution containing CuCl2 can selectively dissolve valuable metals from chalcopyrite and leave minerals with little economic value or penalty elements in the residue. The extraction of Cu within a given length of time in this system was shown to be faster than that of conventional methods. Chalcopyrite has been recognized as the most refractory copper mineral when treated using conventional sulfate aqueous leaching systems. One of the advantages of using DMSO as a solvent is its high boiling point, which allows for a high operating temperature at ambient pressure, leading to a high dissolution rate.
The mechanism of chalcopyrite leaching using a DMSO solution was investigated. Chalcopyrite is oxidized by the cupric ion, which is enhanced by the presence of oxygen below 373 K. The leaching of chalcopyrite takes place depending on the stoichiometric ratio of Cu in the concentrate to the initial cupric ion in the DMSO solution at 413 K because of the low dissolution of oxygen in the DMSO solution.
Data Availability
The data that support the findings of this study are available from the corresponding author, Y.M., upon reasonable request.
References
Khoshkhoo M, Dopson M, Shchukarev A, Sandström Å (2014) Chalcopyrite leaching and bioleaching: an X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 149:220–227
Khoshkhoo M, Dopson M, Engström F, Sandström Å (2017) New insights into the influence of redox potential on chalcopyrite leaching behavior. Miner Eng 100:9–16
Dreisinger D (2006) Copper leaching from primary sulfides: options for biological and chemical extraction of copper. Hydrometallurgy 83:10–20
Li Y, Kawashima N, Li J, Chandra AP, Gerson AR (2013) A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv Colloid Interf Sci 197-198:1–32
Solis-Marcial OJ, Lapidus GT (2013) Improvement of chalcopyrite dissolution in acid media using polar organic solvents. Hydrometallurgy 131:120–126
Solís-Marcíal OJ, Lapidus GT (2014) Chalcopyrite leaching in alcoholic acid media. Hydrometallurgy 147-148:54–58
Ruiz-Sánchez A, Lapidus GT (2017) Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media. Hydrometallurgy 169:192–200
Izutsu K (2009) Electrochemistry in nonaqueous solutions, 2nd revised and enlarged edition. Wiley-VCH, Weinheim
Yoshimura A, Takai M, Matsuno Y (2014) Novel process for recycling gold from secondary sources: leaching of gold by dimethyl sulfoxide solutions containing copper bromide and precipitation with water. Hydrometallurgy 149:177–182
Chandra AP, Gerson AR (2010) The mechanisms of pyrite oxidation and leaching: a fundamental perspective. Surf Sci Rep 65(9):293–315
Elleb M, Meullemeestre J, Schwing-Weill MJ, Vierling F (1982) Spectrophotometric study of copper(II) chloride complexes in propylene carbonate and in dimethyl sulfoxide. Inorg Chem 21:1477–1483
Jeschke S, Johansson P (2017) Predicting solubility of sulfur: a COSMO-RS based approach to investigate electrolytes for Li-S batteries. Chem Eur J 23(38):9130–9136
Ruiz MC, Montes KS, Padilla R (2011) Chalcopyrite leaching in sulfate–chloride media at ambient pressure. Hydrometallurgy 109:37–42
Nazari G, Dixon GD, Dreisinger D (2011) Enhancing the kinetics of chalcopyrite leaching in the Galvanox (TM) process. Hydrometallurgy 105:251–258. https://doi.org/10.1016/j.hydromet.2010.10.013
Nazari G, Dixon GD, Dreisinger D (2012) The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the Galvanox™ process. Hydrometallurgy 113-114:122–130. https://doi.org/10.1016/j.hydromet.2011.12.011
Mulligan M, Chaiko D, Baczek F, Rocks S, Eyzaguirre C, Dickinson C, Klepper R (2017) The FLSmidth® rapid oxidative leach (ROL) process: a mechano-chemical approach and industrial applications for rapid metal sulfide dissolution. J South Afr Inst Min Metall 117(8):741–747
Sawyer DT, Chlerlcato G Jr, Angells CT, Nanni EJ Jr, Tsuchiya T (1982) Effects of media and electrode materials on the electrochemical reduction of dioxygen. Anal Chem 54:1720–1724
Yoshimura A, Umehara K, Takai M, Matsuno Y (2015) Development of recycling system of precious metals and rare metals using DMSO solvent containing CuBr2. J Japan Inst Met Mater 79:41–48
Benari MD, Hefter GT, Parker AJ (1983) Anodic dissolution of silver, copper, palladium and gold in dimethylsulfoxide–halide solution. Hydrometallurgy 10:367–389
Funding
This work was supported by JSPS KAKENHI, Japan [grant number 19H02488].
Author information
Authors and Affiliations
Contributions
Conceptualization, Hidekazu Kato and Yasunari Matsuno; Methodology, Kota Takatori, Hidekazu Kato and Yasunari Matsuno; Software, Kota Takatori; Validation, Kota Takatori, Hidekazu Kato, Akihiro Yoshimura and Yasunari Matsuno; Formal Analysis, Kota Takatori and Akihiro Yoshimura; Investigation, Kota Takatori, A.Y. and Y.M.; Resources, Hidekazu Kato; Data Curation, Kota Takatori; Writing – Original Draft Preparation, Kota Takatori, Akihiro Yoshimura and Yasunari Matsuno; Writing – Review & Editing, Kota Takatori, Akihiro Yoshimura and Yasunari Matsuno; Visualization, Kota Takatori and Akihiro Yoshimura; Supervision, Hidekazu Kato, Akihiro Yoshimura and Yasunari Matsuno; Project Administration, Yasunari Matsuno; Funding Acquisition, Yasunari Matsuno.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 3464 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takatori, K., Kato, H., Yoshimura, A. et al. Chalcopyrite Leaching in a Dimethyl Sulfoxide Solution Containing Copper Chloride. Mining, Metallurgy & Exploration 38, 1477–1485 (2021). https://doi.org/10.1007/s42461-021-00400-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00400-3