Abstract
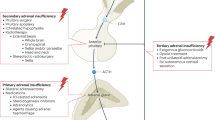
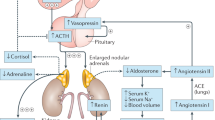
Adrenal insufficiency (AI) is a condition characterized by an absolute or relative deficiency of adrenal cortisol production. Primary AI (PAI) is rare and is caused by direct adrenal failure. Secondary AI (SAI) is more frequent and is caused by diseases affecting the pituitary, whereas in tertiary AI (TAI), the hypothalamus is affected. The most prevalent form is TAI owing to exogenous glucocorticoid use. Symptoms of AI are non-specific, often overlooked or misdiagnosed, and are related to the lack of cortisol, adrenal androgen precursors and aldosterone (especially in PAI). Diagnosis is based on measurement of the adrenal corticosteroid hormones, their regulatory peptide hormones and stimulation tests. The goal of therapy is to establish a hormone replacement regimen that closely mimics the physiological diurnal cortisol secretion pattern, tailored to the patient’s daily needs. This Primer provides insights into the epidemiology, mechanisms and management of AI during pregnancy as well as challenges of long-term management. In addition, the importance of identifying life-threatening adrenal emergencies (acute AI and adrenal crisis) is highlighted and strategies for prevention, which include patient education, glucocorticoid emergency cards and injection kits, are described.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Arlt, W. & Allolio, B. Adrenal insufficiency. Lancet 361, 1881–1893 (2003).
Ross, I. L. & Levitt, N. S. Addison’s disease symptoms–a cross sectional study in urban South Africa. PLoS ONE 8, e53526 (2013).
Erichsen, M. M. et al. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J. Clin. Endocrinol. Metab. 94, 4882–4890 (2009).
Afreen, B., Khan, K. A. & Riaz, A. Adrenal insufficiency in Pakistani HIV infected patients. J. Ayub Med. Coll. Abbottabad 29, 428–431 (2017).
Mofokeng, T. R. P., Beshyah, S. A., Mahomed, F., Ndlovu, K. C. Z. & Ross, I. L. Significant barriers to diagnosis and management of adrenal insufficiency in Africa. Endocr. Connect. 9, 445–456 (2020).
Odeniyi, I. A., Fasanmade, O. A., Ajala, M. O. & Ohwovoriole, A. E. Adrenocortical function in Nigerians with human immunodeficiency virus infection. Ghana. Med. J. 47, 171–177 (2013).
Tripathy, S. K., Agrawala, R. K. & Baliarsinha, A. K. Endocrine alterations in HIV-infected patients. Indian. J. Endocrinol. Metab. 19, 143–147 (2015).
Broersen, L. H., Pereira, A. M., Jorgensen, J. O. & Dekkers, O. M. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 100, 2171–2180 (2015).
Bleicken, B., Hahner, S., Ventz, M. & Quinkler, M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am. J. Med. Sci. 339, 525–531 (2010).
Allolio, B. Extensive expertise in endocrinology adrenal crisis. Eur. J. Endocrinol. 172, R115–R124 (2015).
Hahner, S. et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J. Clin. Endocrinol. Metab. 100, 407–416 (2015).
Puar, T. H., Stikkelbroeck, N. M., Smans, L. C., Zelissen, P. M. & Hermus, A. R. Adrenal crisis: still a deadly event in the 21st century. Am. J. Med. 129, 339 e331–339 e339 (2016).
Rushworth, R. L., Torpy, D. J. & Falhammar, H. Adrenal crises: perspectives and research directions. Endocrine 55, 336–345 (2017).
Smans, L. C., Van der Valk, E. S., Hermus, A. R. & Zelissen, P. M. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin. Endocrinol. 84, 17–22 (2016).
Bornstein, S. R. et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 364–389 (2016). Practice guidelines on the management of adrenal insufficiency from an international expert panel.
Dunlop, D. Eighty-six cases of Addison’s disease. Br. Med. J. 2, 887–891 (1963).
Laureti, S., Vecchi, L., Santeusanio, F. & Falorni, A. Is the prevalence of Addison’s disease underestimated? J. Clin. Endocrinol. Metab. 84, 1762 (1999).
Hellesen, A., Bratland, E. & Husebye, E. S. Autoimmune Addison’s disease – an update on pathogenesis. Ann. Endocrinol. 79, 157–163 (2018).
Mason, A. S., Meade, T. W., Lee, J. A. & Morris, J. N. Epidemiological and clinical picture of Addison’s disease. Lancet 2, 744–747 (1968).
Olafsson, A. S. & Sigurjonsdottir, H. A. Increasing prevalence of Addison disease: results from a nationwide study. Endocr. Pract. 22, 30–35 (2016).
Bjornsdottir, S. et al. Drug prescription patterns in patients with Addison’s disease: a Swedish population-based cohort study. J. Clin. Endocrinol. Metab. 98, 2009–2018 (2013).
Meyer, G., Neumann, K., Badenhoop, K. & Linder, R. Increasing prevalence of Addison’s disease in German females: health insurance data 2008-2012. Eur. J. Endocrinol. 170, 367–373 (2014).
Stewart, P. M. et al. Exploring inpatient hospitalizations and morbidity in patients with adrenal insufficiency. J. Clin. Endocrinol. Metab. 101, 4843–4850 (2016).
Willis, A. C. & Vince, F. P. The prevalence of Addison’s disease in Coventry, UK. Postgrad. Med. J. 73, 286–288 (1997).
Chantzichristos, D. et al. Incidence, prevalence and seasonal onset variation of Addison’s disease among persons with type 1 diabetes mellitus: nationwide, matched cohort studies. Eur. J. Endocrinol. 178, 113–120 (2018). A nationwide, matched, observational cohort study investigating the incidence and prevalence of primary adrenal insufficiency in Sweden.
Betterle, C., Dal Pra, C., Mantero, F. & Zanchetta, R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr. Rev. 23, 327–364 (2002).
Laureti, S. et al. Levels of adrenocortical autoantibodies correlate with the degree of adrenal dysfunction in subjects with preclinical Addison’s disease. J. Clin. Endocrinol. Metab. 83, 3507–3511 (1998).
Herndon, J., Nadeau, A. M., Davidge-Pitts, C. J., Young, W. F. & Bancos, I. Primary adrenal insufficiency due to bilateral infiltrative disease. Endocrine 62, 721–728 (2018).
Merke, D. P. & Auchus, R. J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N. Engl. J. Med. 383, 1248–1261 (2020).
White, P. C. & Speiser, P. W. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr. Rev. 21, 245–291 (2000).
Bancos, I., Hahner, S., Tomlinson, J. & Arlt, W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 3, 216–226 (2015).
Michels, A. W. & Gottlieb, P. A. Autoimmune polyglandular syndromes. Nat. Rev. Endocrinol. 6, 270–277 (2010).
Husebye, E. S., Anderson, M. S. & Kampe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 2543–2544 (2018).
Dalin, F. et al. Clinical and immunological characteristics of autoimmune Addison disease: a nationwide Swedish multicenter study. J. Clin. Endocrinol. Metab. 102, 379–389 (2017).
Reato, G. et al. Premature ovarian failure in patients with autoimmune Addison’s disease: clinical, genetic, and immunological evaluation. J. Clin. Endocrinol. Metab. 96, E1255–E1261 (2011).
Charmandari, E., Nicolaides, N. C. & Chrousos, G. P. Adrenal insufficiency. Lancet 383, 2152–2167 (2014).
Regal, M., Paramo, C., Sierra, S. M. & Garcia-Mayor, R. V. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin. Endocrinol. 55, 735–740 (2001).
Bates, A. S., Van’t Hoff, W., Jones, P. J. & Clayton, R. N. The effect of hypopituitarism on life expectancy. J. Clin. Endocrinol. Metab. 81, 1169–1172 (1996).
Tomlinson, J. W. et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 357, 425–431 (2001). A prospective study including 1,014 patients with hypopituitarism investigating the total and specific-cause mortality compared with an age-matched and sex-matched UK population.
Carosi, G. et al. Hypothalamic-pituitary axis in non-functioning pituitary adenomas: focus on the prevalence of isolated central hypoadrenalism. Neuroendocrinology 102, 267–273 (2015).
Appelman-Dijkstra, N. M. et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 96, 2330–2340 (2011).
Jenkins, P. J., Bates, P., Carson, M. N., Stewart, P. M. & Wass, J. A. Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor-I in patients with acromegaly. J. Clin. Endocrinol. Metab. 91, 1239–1245 (2006).
Knappe, U. J. et al. Fractionated radiotherapy and radiosurgery in acromegaly: analysis of 352 patients from the German Acromegaly Registry. Eur. J. Endocrinol. 182, 275–284 (2020).
Imber, B. S., Lee, H. S., Kunwar, S., Blevins, L. S. & Aghi, M. K. Hypophysitis: a single-center case series. Pituitary 18, 630–641 (2015).
Wang, S. et al. Primary lymphocytic hypophysitis: clinical characteristics and treatment of 50 cases in a single centre in China over 18 years. Clin. Endocrinol. 87, 177–184 (2017).
Turcu, A. F. et al. Pituitary stalk lesions: the Mayo Clinic experience. J. Clin. Endocrinol. Metab. 98, 1812–1818 (2013).
Lu, J., Li, L., Lan, Y., Liang, Y. & Meng, H. Immune checkpoint inhibitor-associated pituitary-adrenal dysfunction: a systematic review and meta-analysis. Cancer Med. 8, 7503–7515 (2019).
Barroso-Sousa, R. et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 4, 173–182 (2018).
Chang, L. S. et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 40, 17–65 (2019).
Li, T. et al. Prevalence of opioid-induced adrenal insufficiency in patients taking chronic opioids. J. Clin. Endocrinol. Metab. 105, e3766–e3775 (2020).
de Vries, F. et al. Opioids and their endocrine effects: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 105, 1020–1029 (2020).
Saeed, Z. I., Bancos, I. & Donegan, D. Current knowledge and practices of heath care professionals on opioid-induced adrenal insufficiency. Endocr. Pract. 25, 1012–1021 (2019).
Woods, C. P. et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur. J. Endocrinol. 173, 633–642 (2015).
Schlaghecke, R., Kornely, E., Santen, R. T. & Ridderskamp, P. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N. Engl. J. Med. 326, 226–230 (1992).
Mebrahtu, T. F. et al. Dose dependency of iatrogenic glucocorticoid excess and adrenal insufficiency and mortality: a cohort study in England. J. Clin. Endocrinol. Metab. 104, 3757–3767 (2019).
Pofi, R. et al. The short Synacthen (corticotropin) test can be used to predict recovery of hypothalamo-pituitary-adrenal axis function. J. Clin. Endocrinol. Metab. 103, 3050–3059 (2018). A retrospective analysis of 776 patients with reversible causes of AI who had at least two short Synacthen tests performed to predict recovery of HPA axis function.
Joseph, R. M., Hunter, A. L., Ray, D. W. & Dixon, W. G. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin. Arthritis Rheum. 46, 133–141 (2016).
Leong, S. H., Shander, S. & Ratnasingam, J. Predicting recovery of the hypothalamic-pituitary-adrenal axis after prolonged glucocorticoid use. Endocr. Pract. 24, 14–20 (2018).
Odenwald, B. et al. Children with classic congenital adrenal hyperplasia experience salt loss and hypoglycemia: evaluation of adrenal crises during the first 6 years of life. Eur. J. Endocrinol. 174, 177–186 (2016).
Reisch, N. et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur. J. Endocrinol. 167, 35–42 (2012).
Ritzel, K. et al. Clinical review: outcome of bilateral adrenalectomy in Cushing’s syndrome: a systematic review. J. Clin. Endocrinol. Metab. 98, 3939–3948 (2013).
Meyer, G., Badenhoop, K. & Linder, R. Addison’s disease with polyglandular autoimmunity carries a more than 2.5-fold risk for adrenal crises: German health insurance data 2010-2013. Clin. Endocrinol. 85, 347–353 (2016).
Papierska, L. & Rabijewski, M. Delay in diagnosis of adrenal insufficiency is a frequent cause of adrenal crisis. Int. J. Endocrinol. 2013, 482370 (2013).
Gidlof, S. et al. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 1, 35–42 (2013).
Bergthorsdottir, R., Leonsson-Zachrisson, M., Oden, A. & Johannsson, G. Premature mortality in patients with Addison’s disease: a population-based study. J. Clin. Endocrinol. Metab. 91, 4849–4853 (2006). A population-based, retrospective, observational study from Sweden showing that the risk ratio for death is more than twofold higher in patients with Addison disease than in the background population.
Bensing, S. et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin. Endocrinol. 69, 697–704 (2008).
Erichsen, M. M. et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur. J. Endocrinol. 160, 233–237 (2009).
Falhammar, H. et al. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 99, E2715–E2721 (2014).
Hahner, S. et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur. J. Endocrinol. 162, 597–602 (2010).
El-Maouche, D. et al. Longitudinal assessment of illnesses, stress dosing, and illness sequelae in patients with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 103, 2336–2345 (2018).
Rushworth, R. L., Chrisp, G. L., Dean, B., Falhammar, H. & Torpy, D. J. Hospitalisation in children with adrenal insufficiency and hypopituitarism: is there a differential burden between boys and girls and between age groups? Horm. Res. Paediatr. 88, 339–346 (2017).
Rushworth, R. L., Falhammar, H., Munns, C. F., Maguire, A. M. & Torpy, D. J. Hospital admission patterns in children with CAH: admission rates and adrenal crises decline with age. Int. J. Endocrinol. 2016, 5748264 (2016).
Rushworth, R. L. & Torpy, D. J. A descriptive study of adrenal crises in adults with adrenal insufficiency: increased risk with age and in those with bacterial infections. BMC Endocr. Disord. 14, 79 (2014).
Rushworth, R. L. & Torpy, D. J. Modern hydrocortisone replacement regimens in adrenal insufficiency patients and the risk of adrenal crisis. Horm. Metab. Res. 47, 637–642 (2015).
Rushworth, R. L. & Torpy, D. J. Adrenal insufficiency in Australia: is it possible that the use of lower dose, short-acting glucocorticoids has increased the risk of adrenal crises? Horm. Metab. Res. 47, 427–432 (2015).
Okamoto, M. The metabolism of cortisol in hyperthyroidism and Cushing’s syndrome. Endocrinol. Jpn. 10, 159–168 (1963).
Fonseca, V., Brown, R., Hochhauser, D., Ginsburg, J. & Havard, C. W. Acute adrenal crisis precipitated by thyroxine. Br. Med. J. 292, 1185–1186 (1986).
Rushworth, R. L., Chrisp, G. L. & Torpy, D. J. Glucocorticoid-induced adrenal insufficiency: a study of the incidence in hospital patients and a review of peri-operative management. Endocr. Pract. 24, 437–445 (2018).
Rushworth, R. L., Slobodian, P. & Torpy, D. J. Interruptions to supply of high-dose hydrocortisone tablets and the incidence of adrenal crises. Clin. Endocrinol. 83, 999–1000 (2015).
Gjerstad, J. K., Lightman, S. L. & Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21, 403–416 (2018).
Lightman, S. L. & Conway-Campbell, B. L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718 (2010).
Roelfsema, F., Aoun, P. & Veldhuis, J. D. Pulsatile cortisol feedback on ACTH secretion is mediated by the glucocorticoid receptor and modulated by gender. J. Clin. Endocrinol. Metab. 101, 4094–4102 (2016).
Walker, J. J., Terry, J. R. & Lightman, S. L. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc. Biol. Sci. 277, 1627–1633 (2010).
Lightman, S. L., Birnie, M. T. & Conway-Campbell, B. L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 41, 470–490 (2020).
Oster, H. et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 38, 3–45 (2017).
Caratti, G. et al. Glucocorticoid receptor function in health and disease. Clin. Endocrinol. 83, 441–448 (2015).
Chrousos, G. P. in Endocrinology 7th edition (eds Jameson, J. L. & De Groot, L.) 1727–1740.e5 (Elsevier, 2016).
Stavreva, D. A. et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat. Cell Biol. 11, 1093–1102 (2009). This study showed that an ultradian administration of glucocorticoids induces cyclic GR-mediated transcriptional regulation in cultured cells and in animal models.
Kalafatakis, K. et al. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc. Natl Acad. Sci. USA 115, E4091–E4100 (2018).
Chan, W. L., Carrell, R. W., Zhou, A. & Read, R. J. How changes in affinity of corticosteroid-binding globulin modulate free cortisol concentration. J. Clin. Endocrinol. Metab. 98, 3315–3322 (2013).
Henley, D., Lightman, S. & Carrell, R. Cortisol and CBG – Getting cortisol to the right place at the right time. Pharmacol. Ther. 166, 128–135 (2016).
Stowasser, M., Ahmed, A. H., Pimenta, E., Taylor, P. J. & Gordon, R. D. Factors affecting the aldosterone/renin ratio. Horm. Metab. Res. 44, 170–176 (2012).
Atlas, S. A. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 13, 9–20 (2007).
Tucci, J. R., Espiner, E. A., Jagger, P. I., Pauk, G. L. & Lauler, D. P. ACTH stimulation of aldosterone secretion in normal subjects and in patients with chronic adrenocortical insufficiency. J. Clin. Endocrinol. Metab. 27, 568–575 (1967).
Kem, D. C. et al. Plasma aldosterone response to ACTH in primary aldosteronism and in patients with low renin hypertension. J. Clin. Endocrinol. Metab. 46, 552–560 (1978).
Bollag, W. B. Regulation of aldosterone synthesis and secretion. Compr. Physiol. 4, 1017–1055 (2014).
Mitchell, A. L. & Pearce, S. H. Autoimmune Addison disease: pathophysiology and genetic complexity. Nat. Rev. Endocrinol. 8, 306–316 (2012).
Winqvist, O., Karlsson, F. A. & Kampe, O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet 339, 1559–1562 (1992).
Naletto, L. et al. The natural history of autoimmune Addison’s disease from the detection of autoantibodies to development of the disease: a long follow-up study on 143 patients. Eur. J. Endocrinol. 180, 223–234 (2019).
Dawoodji, A. et al. High frequency of cytolytic 21-hydroxylase-specific CD8+ T cells in autoimmune Addison’s disease patients. J. Immunol. 193, 2118–2126 (2014).
Bratland, E., Skinningsrud, B., Undlien, D. E., Mozes, E. & Husebye, E. S. T cell responses to steroid cytochrome P450 21-hydroxylase in patients with autoimmune primary adrenal insufficiency. J. Clin. Endocrinol. Metab. 94, 5117–5124 (2009).
Eriksson, D. et al. Common genetic variation in the autoimmune regulator (AIRE) locus is associated with autoimmune Addison’s disease in Sweden. Sci. Rep. 8, 8395 (2018).
Skov, J. et al. Heritability of Addison’s disease and prevalence of associated autoimmunity in a cohort of 112,100 Swedish twins. Endocrine 58, 521–527 (2017).
Loeb, R. F. Chemical changes in the blood in Addison’s disease. Science 76, 420–421 (1932).
Han, T. S., Walker, B. R., Arlt, W. & Ross, R. J. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat. Rev. Endocrinol. 10, 115–124 (2014).
Kemp, S., Huffnagel, I. C., Linthorst, G. E., Wanders, R. J. & Engelen, M. Adrenoleukodystrophy – neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 12, 606–615 (2016).
Laureti, S. et al. X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison’s disease in young adult male patients. J. Clin. Endocrinol. Metab. 81, 470–474 (1996).
Bezman, L. & Moser, H. W. Incidence of X-linked adrenoleukodystrophy and the relative frequency of its phenotypes. Am. J. Med. Genet. 76, 415–419 (1998).
Hannah-Shmouni, F. & Stratakis, C. A. An overview of inborn errors of metabolism manifesting with primary adrenal insufficiency. Rev. Endocr. Metab. Disord. 19, 53–67 (2018).
Narumi, S. Rare monogenic causes of primary adrenal insufficiency. Curr. Opin. Endocrinol. Diabetes Obes. 25, 172–177 (2018).
Tanriverdi, F. et al. Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr. Rev. 36, 305–342 (2015).
Gubbi, S., Hannah-Shmouni, F., Stratakis, C. A. & Koch, C. A. Primary hypophysitis and other autoimmune disorders of the sellar and suprasellar regions. Rev. Endocr. Metab. Disord. 19, 335–347 (2018).
Faje, A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 19, 82–92 (2016).
Caturegli, P. et al. Autoimmune hypophysitis. Endocr. Rev. 26, 599–614 (2005).
AbdelRazek, M. A., Venna, N. & Stone, J. H. IgG4-related disease of the central and peripheral nervous systems. Lancet Neurol. 17, 183–192 (2018).
Lamprecht, A., Sorbello, J., Jang, C., Torpy, D. J. & Inder, W. J. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur. J. Endocrinol. 179, 353–362 (2018).
Fraser, L. A. et al. Oral opioids for chronic non-cancer pain: higher prevalence of hypogonadism in men than in women. Exp. Clin. Endocrinol. Diabetes 117, 38–43 (2009).
Fountas, A., Van Uum, S. & Karavitaki, N. Opioid-induced endocrinopathies. Lancet Diabetes Endocrinol. 8, 68–80 (2020).
Pekic, S. & Popovic, V. Diagnosis of endocrine disease: expanding the cause of hypopituitarism. Eur. J. Endocrinol. 176, R269–R282 (2017).
Higham, C. E., Johannsson, G. & Shalet, S. M. Hypopituitarism. Lancet 388, 2403–2415 (2016).
Wu, W. et al. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat. Genet. 18, 147–149 (1998).
Jullien, N. et al. Clinical lessons learned in constitutional hypopituitarism from two decades of experience in a large international cohort. Clin. Endocrinol. 94, 277–289 (2021).
Tutunculer, F., Saka, N., Arkaya, S. C., Abbasoglu, S. & Bas, F. Evaluation of adrenomedullary function in patients with congenital adrenal hyperplasia. Horm. Res. 72, 331–336 (2009).
Morita, S. et al. Reduced epinephrine reserve in response to insulin-induced hypoglycemia in patients with pituitary adenoma. Eur. J. Endocrinol. 157, 265–270 (2007).
Merke, D. P. et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N. Engl. J. Med. 343, 1362–1368 (2000).
Bornstein, S. R., Breidert, M., Ehrhart-Bornstein, M., Kloos, B. & Scherbaum, W. A. Plasma catecholamines in patients with Addison’s disease. Clin. Endocrinol. 42, 215–218 (1995).
Mpoy, M. & Kolanowski, J. Urinary catecholamine excretion in patients with secondary adrenocortical insufficiency. J. Endocrinol. Invest. 9, 253–255 (1986).
Kim, M. S. et al. Decreased adrenomedullary function in infants with classical congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 99, E1597–E1601 (2014).
Geiger, A. M., Pitts, K. P., Feldkamp, J., Kirschbaum, C. & Wolf, J. M. Cortisol-dependent stress effects on cell distribution in healthy individuals and individuals suffering from chronic adrenal insufficiency. Brain Behav. Immun. 50, 241–248 (2015).
Wurtman, R. J. & Axelrod, J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J. Biol. Chem. 241, 2301–2305 (1966).
Ehrhart-Bornstein, M., Hinson, J. P., Bornstein, S. R., Scherbaum, W. A. & Vinson, G. P. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr. Rev. 19, 101–143 (1998).
To, T. T. et al. Pituitary-interrenal interaction in zebrafish interrenal organ development. Mol. Endocrinol. 21, 472–485 (2007).
Weise, M. et al. Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glucose elevation in response to high-intensity exercise. J. Clin. Endocrinol. Metab. 89, 591–597 (2004).
Elbelt, U., Hahner, S. & Allolio, B. Altered insulin requirement in patients with type 1 diabetes and primary adrenal insufficiency receiving standard glucocorticoid replacement therapy. Eur. J. Endocrinol. 160, 919–924 (2009).
Orentreich, N., Brind, J. L., Rizer, R. L. & Vogelman, J. H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J. Clin. Endocrinol. Metab. 59, 551–555 (1984).
Korth-Schutz, S., Levine, L. S. & New, M. I. Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. J. Clin. Endocrinol. Metab. 42, 117–124 (1976).
Lebbe, M. et al. The steroid metabolome in the isolated ovarian follicle and its response to androgen exposure and antagonism. Endocrinology 158, 1474–1485 (2017).
Gleicher, N., Weghofer, A. & Barad, D. H. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment? Reprod. Biol. Endocrinol. 9, 116 (2011).
Lang, K., Burger-Stritt, S. & Hahner, S. Is DHEA replacement beneficial in chronic adrenal failure? Best Pract. Res. Clin. Endocrinol. Metab. 29, 25–32 (2015).
Labrie, F. et al. Structure, regulation and role of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase and aromatase enzymes in the formation of sex steroids in classical and peripheral intracrine tissues. Baillieres Clin. Endocrinol. Metab. 8, 451–474 (1994).
Pluchino, N. et al. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol. 145, 273–280 (2015).
Arlt, W. et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N. Engl. J. Med. 341, 1013–1020 (1999). A double-blind clinical study investigating DHEA in women with AI demonstrating improved well-being and sexuality.
Sapolsky, R. M., Romero, L. M. & Munck, A. U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (2000).
Besse, J. C. & Bass, A. D. Potentiation by hydrocortisone of responses to catecholamines in vascular smooth muscle. J. Pharmacol. Exp. Ther. 154, 224–238 (1966).
Kalsner, S. Mechanism of hydrocortisone potentiation of responses to epinephrine and norepinephrine in rabbit aorta. Circ. Res. 24, 383–395 (1969).
Prete, A. et al. Prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J. Clin. Endocrinol. Metab. 105, 2262–2274 (2020). This study provides an evidence base on the steroidogenic response of the adrenal cortex in case of major stress (major trauma, sepsis and combat stress).
Udelsman, R. et al. Adaptation during surgical stress. A reevaluation of the role of glucocorticoids. J. Clin. Invest. 77, 1377–1381 (1986).
Quinkler, M., Oelkers, W., Remde, H. & Allolio, B. Mineralocorticoid substitution and monitoring in primary adrenal insufficiency. Best Pract. Res. Clin. Endocrinol. Metab. 29, 17–24 (2015).
Munck, A., Guyre, P. M. & Holbrook, N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 5, 25–44 (1984).
Besedovsky, H., del Rey, A., Sorkin, E. & Dinarello, C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233, 652–654 (1986).
Barber, A. E. et al. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 150, 1999–2006 (1993).
Morrow, L. E., McClellan, J. L., Conn, C. A. & Kluger, M. J. Glucocorticoids alter fever and IL-6 responses to psychological stress and to lipopolysaccharide. Am. J. Physiol. 264, R1010–R1016 (1993).
Koniaris, L. G., Wand, G. & Wright, T. M. TNF mediates a murine model of Addison’s crisis. Shock 15, 29–34 (2001).
Boonen, E. et al. Reduced cortisol metabolism during critical illness. N. Engl. J. Med. 368, 1477–1488 (2013).
Husebye, E. S. et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J. Intern. Med. 275, 104–115 (2014).
Buonocore, F., McGlacken-Byrne, S. M., Del Valle, I. & Achermann, J. C. Current insights into adrenal insufficiency in the newborn and young infant. Front. Pediatr. 8, 619041 (2020).
Saevik, A. B. et al. Clues for early detection of autoimmune Addison’s disease – myths and realities. J. Intern. Med. 283, 190–199 (2018).
El-Farhan, N. et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin. Endocrinol. 78, 673–680 (2013).
Ueland, G. A. et al. The short cosyntropin test revisited: new normal reference range using LC-MS/MS. J. Clin. Endocrinol. Metab. 103, 1696–1703 (2018).
Dickstein, G. et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J. Clin. Endocrinol. Metab. 72, 773–778 (1991).
May, M. E. & Carey, R. M. Rapid adrenocorticotropic hormone test in practice. retrospective review. Am. J. Med. 79, 679–684 (1985).
Oelkers, W. The role of high- and low-dose corticotropin tests in the diagnosis of secondary adrenal insufficiency. Eur. J. Endocrinol. 139, 567–570 (1998).
Lutz, A. et al. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur. J. Endocrinol. 143, 91–97 (2000).
Mao, J. J., Dages, K. N., Suresh, M. & Bancos, I. Presentation, disease progression and outcomes of adrenal gland metastases. Clin. Endocrinol. 93, 546–554 (2020).
Petersenn, S., Honegger, J. & Quinkler, M. National German audit of diagnosis, treatment, and teaching in secondary adrenal insufficiency. Horm. Metab. Res. 49, 580–588 (2017).
Schmidt, I. L., Lahner, H., Mann, K. & Petersenn, S. Diagnosis of adrenal insufficiency: evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. J. Clin. Endocrinol. Metab. 88, 4193–4198 (2003).
Grossman, A. B. The diagnosis and management of central hypoadrenalism. J. Clin. Endocrinol. Metab. 95, 4855–4863 (2010).
Erturk, E., Jaffe, C. A. & Barkan, A. L. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J. Clin. Endocrinol. Metab. 83, 2350–2354 (1998).
Hurel, S. J. et al. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin. Endocrinol. 44, 141–146 (1996).
Reynolds, R. M., Stewart, P. M., Seckl, J. R. & Padfield, P. L. Assessing the HPA axis in patients with pituitary disease: a UK survey. Clin. Endocrinol. 64, 82–85 (2006).
Mukherjee, J. J. et al. A comparison of the insulin tolerance/glucagon test with the short ACTH stimulation test in the assessment of the hypothalamo-pituitary-adrenal axis in the early post-operative period after hypophysectomy. Clin. Endocrinol. 47, 51–60 (1997).
Ospina, N. S. et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 101, 427–434 (2016).
Steiner, H., Bahr, V., Exner, P. & Oelkers, P. W. Pituitary function tests: comparison of ACTH and 11-deoxy-cortisol responses in the metyrapone test and with the insulin hypoglycemia test. Exp. Clin. Endocrinol. 102, 33–38 (1994).
Berneis, K. et al. Combined stimulation of adrenocorticotropin and compound-S by single dose metyrapone test as an outpatient procedure to assess hypothalamic-pituitary-adrenal function. J. Clin. Endocrinol. Metab. 87, 5470–5475 (2002).
Giordano, R. et al. Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin. Endocrinol. 68, 935–941 (2008).
Hamrahian, A. H. et al. Revised GH and cortisol cut-points for the glucagon stimulation test in the evaluation of GH and hypothalamic-pituitary-adrenal axes in adults: results from a prospective randomized multicenter study. Pituitary 19, 332–341 (2016).
Deutschbein, T., Unger, N., Mann, K. & Petersenn, S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur. J. Endocrinol. 160, 9–16 (2009).
Debono, M. et al. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J. Clin. Endocrinol. Metab. 101, 1469–1477 (2016).
Thil’en, A. et al. Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden. Pediatrics 101, E11 (1998).
Kishore Kumar, R., Das, H. & Kini, P. Newborn screening for congenital adrenal hyperplasia in India: what do we need to watch out for? J. Obstet. Gynaecol. India 66, 415–419 (2016).
Yanase, T. et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [Opinion]. Endocr. J. 63, 765–784 (2016).
Woodcock, T. et al. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia 75, 654–663 (2020).
Grossman, A., Johannsson, G., Quinkler, M. & Zelissen, P. Therapy of endocrine disease: perspectives on the management of adrenal insufficiency: clinical insights from across Europe. Eur. J. Endocrinol. 169, R165–R175 (2013).
Debono, M. et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. 94, 1548–1554 (2009).
Hahner, S. et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J. Clin. Endocrinol. Metab. 92, 3912–3922 (2007). A cross-sectional study showing that patients with AI on current standard replacement suffer from significantly impaired health-related subjective health status, irrespective of origin of disease or concomitant disease.
Johannsson, G. et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J. Clin. Endocrinol. Metab. 97, 473–481 (2012). A randomized trial comparing thrice-daily conventional hydrocortisone replacement with once-daily dual-release hydrocortisone.
European Medicines Agency. Summary of Product Characteristics: Plenadren. https://www.ema.europa.eu/en/medicines/human/EPAR/plenadren (2018).
Giordano, R. et al. Improvement of anthropometric and metabolic parameters, and quality of life following treatment with dual-release hydrocortisone in patients with Addison’s disease. Endocrine 51, 360–368 (2016).
Quinkler, M., Miodini Nilsen, R., Zopf, K., Ventz, M. & Oksnes, M. Modified-release hydrocortisone decreases BMI and HbA1c in patients with primary and secondary adrenal insufficiency. Eur. J. Endocrinol. 172, 619–626 (2015).
Isidori, A. M. et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 6, 173–185 (2018).
Whittle, E. & Falhammar, H. Glucocorticoid regimens in the treatment of congenital adrenal hyperplasia: a systematic review and meta-analysis. J. Endocr. Soc. 3, 1227–1245 (2019).
Lovas, K. & Husebye, E. S. Continuous subcutaneous hydrocortisone infusion in Addison’s disease. Eur. J. Endocrinol. 157, 109–112 (2007).
Gagliardi, L. et al. Continuous subcutaneous hydrocortisone infusion therapy in Addison’s disease: a randomized, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 99, 4149–4157 (2014).
Nella, A. A. et al. A phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 101, 4690–4698 (2016).
Whitaker, M. et al. An oral multiparticulate, modified-release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin. Endocrinol. 80, 554–561 (2014).
Mallappa, A. et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 100, 1137–1145 (2015). Phase II study investigating the modified-release hydrocortisone formulation Chronocort in 16 adults with classic congenital adrenal hyperplasia. Twice-daily Chronocort induced cortisol profiles similar to physiological cortisol secretion.
Merke, D. P. et al. A phase 3 study of a modified-release hydrocortisone in the treatment of congenital adrenal hyperplasia [abstract OR25-02]. J. Endocr. Soc. 4 (Suppl. 1), A107–A108 (2020).
Turcu, A. F. et al. Single-dose study of a corticotropin-releasing factor receptor-1 antagonist in women with 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 101, 1174–1180 (2016).
Saevik, A. B. et al. Residual corticosteroid production in autoimmune Addison disease. J. Clin. Endocrinol. Metab. 105, 2430–2441 (2020).
Pofi, R. et al. Plasma renin measurements are unrelated to mineralocorticoid replacement dose in patients with primary adrenal insufficiency. J. Clin. Endocrinol. Metab. 105, 314–326 (2020).
Oelkers, W., Diederich, S. & Bahr, V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J. Clin. Endocrinol. Metab. 75, 259–264 (1992).
Schultebraucks, K., Wingenfeld, K., Otte, C. & Quinkler, M. The role of fludrocortisone in cognition and mood in patients with primary adrenal insufficiency (Addison’s disease). Neuroendocrinology 103, 315–320 (2016).
Esposito, D., Pasquali, D. & Johannsson, G. Primary adrenal insufficiency: managing mineralocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 103, 376–387 (2018).
Flad, T. M., Conway, J. D., Cunningham, S. K. & McKenna, T. J. The role of plasma renin activity in evaluating the adequacy of mineralocorticoid replacement in primary adrenal insufficiency. Clin. Endocrinol. 45, 529–534 (1996).
Cohen, N., Gilbert, R., Wirth, A., Casley, D. & Jerums, G. Atrial natriuretic peptide and plasma renin levels in assessment of mineralocorticoid replacement in Addison’s disease. J. Clin. Endocrinol. Metab. 81, 1411–1415 (1996).
Oelkers, W. & L’Age, M. Control of mineralocorticoid substitution in Addison’s disease by plasma renin measurement. Klin. Wochenschr. 54, 607–612 (1976).
Smith, S. J. et al. Evidence that patients with Addison’s disease are undertreated with fludrocortisone. Lancet 1, 11–14 (1984).
Chortis, V. et al. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J. Clin. Endocrinol. Metab. 98, 161–171 (2013).
Johannsson, G. et al. Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J. Clin. Endocrinol. Metab. 87, 2046–2052 (2002).
Lovas, K. et al. Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J. Clin. Endocrinol. Metab. 88, 1112–1118 (2003).
van Thiel, S. W. et al. Effects of dehydroepiandrostenedione, superimposed on growth hormone substitution, on quality of life and insulin-like growth factor I in patients with secondary adrenal insufficiency: a randomized, placebo-controlled, cross-over trial. J. Clin. Endocrinol. Metab. 90, 3295–3303 (2005).
Gurnell, E. M. et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 93, 400–409 (2008).
Coles, A. J. et al. Dehydroepiandrosterone replacement in patients with Addison’s disease has a bimodal effect on regulatory (CD4+CD25hi and CD4+FoxP3+) T cells. Eur. J. Immunol. 35, 3694–3703 (2005).
Dhatariya, K., Bigelow, M. L. & Nair, K. S. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes 54, 765–769 (2005).
Srinivasan, M. et al. Effect of dehydroepiandrosterone replacement on lipoprotein profile in hypoadrenal women. J. Clin. Endocrinol. Metab. 94, 761–764 (2009).
Callies, F. et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency: effects on body composition, serum leptin, bone turnover, and exercise capacity. J. Clin. Endocrinol. Metab. 86, 1968–1972 (2001).
Christiansen, J. J. et al. Very short term dehydroepiandrosterone treatment in female adrenal failure: impact on carbohydrate, lipid and protein metabolism. Eur. J. Endocrinol. 152, 77–85 (2005).
Alkatib, A. A. et al. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J. Clin. Endocrinol. Metab. 94, 3676–3681 (2009). Meta-analysis of randomized trials of DHEA in AI showing overall small effects of DHEA on quality of life parameters and sexuality.
Allolio, B., Arlt, W. & Hahner, S. DHEA: why, when, and how much–DHEA replacement in adrenal insufficiency. Ann. Endocrinol. 68, 268–273 (2007). Comprehensive reflection of available evidence on adrenal crisis and discussion of future directions.
Lovas, K. et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. Eur. J. Endocrinol. 160, 993–1002 (2009).
Koetz, K. R., Ventz, M., Diederich, S. & Quinkler, M. Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 97, 85–92 (2012). Systematic assessment of BMD in 122 patients with AI demonstrating normal BMD values on low glucocorticoid replacement doses.
Schulz, J. et al. Reduction in daily hydrocortisone dose improves bone health in primary adrenal insufficiency. Eur. J. Endocrinol. 174, 531–538 (2016).
Bjornsdottir, S. et al. Risk of hip fracture in Addison’s disease: a population-based cohort study. J. Intern. Med. 270, 187–195 (2011).
Ragnarsson, O., Nystrom, H. F. & Johannsson, G. Glucocorticoid replacement therapy is independently associated with reduced bone mineral density in women with hypopituitarism. Clin. Endocrinol. 76, 246–252 (2012).
Mo, D. et al. Fracture risk in adult patients treated with growth hormone replacement therapy for growth hormone deficiency: a prospective observational cohort study. Lancet Diabetes Endocrinol. 3, 331–338 (2015).
Mazziotti, G. et al. Management of endocrine disease: risk of overtreatment in patients with adrenal insufficiency: current and emerging aspects. Eur. J. Endocrinol. 177, R231–R248 (2017).
Falhammar, H., Filipsson Nystrom, H., Wedell, A., Brismar, K. & Thoren, M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur. J. Endocrinol. 168, 331–341 (2013).
Guarnotta, V., Ciresi, A., Pillitteri, G. & Giordano, C. Improved insulin sensitivity and secretion in prediabetic patients with adrenal insufficiency on dual-release hydrocortisone treatment: a 36-month retrospective analysis. Clin. Endocrinol. 88, 665–672 (2018).
Plat, L. et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J. Clin. Endocrinol. Metab. 84, 3082–3092 (1999). This study showed that elevations of evening cortisol levels could contribute to alterations in glucose tolerance, insulin sensitivity and insulin secretion.
Filipsson, H., Monson, J. P., Koltowska-Haggstrom, M., Mattsson, A. & Johannsson, G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J. Clin. Endocrinol. Metab. 91, 3954–3961 (2006). Large Swedish cohort–control study revealing increased risk of ischaemic heart disease with increasing steroid replacement dose in women with autoimmune Addison disease. No increase in cerebrovascular disease risk was detected.
Skov, J., Sundstrom, A., Ludvigsson, J. F., Kampe, O. & Bensing, S. Sex-specific risk of cardiovascular disease in autoimmune Addison disease–a population-based cohort study. J. Clin. Endocrinol. Metab. 104, 2031–2040 (2019).
Falhammar, H. et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J. Clin. Endocrinol. Metab. 100, 3520–3528 (2015).
Quinkler, M. et al. Prednisolone is associated with a worse lipid profile than hydrocortisone in patients with adrenal insufficiency. Endocr. Connect. 6, 1–8 (2017).
Han, T. S. et al. Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur. J. Endocrinol. 168, 887–893 (2013).
Han, T. S. et al. Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin. Endocrinol. 78, 197–203 (2013).
Behan, L. A. et al. Low-dose hydrocortisone replacement is associated with improved arterial stiffness index and blood pressure dynamics in severely adrenocorticotrophin-deficient hypopituitary male patients. Eur. J. Endocrinol. 174, 791–799 (2016).
Werumeus Buning, J. et al. Effects of hydrocortisone on the regulation of blood pressure: results from a randomized controlled trial. J. Clin. Endocrinol. Metab. 101, 3691–3699 (2016).
Ventura, M. et al. The spectrum of pediatric adrenal insufficiency: insights from 34 years of experience. J. Pediatr. Endocrinol. Metab. 32, 721–726 (2019).
Sterns, R. H. Disorders of plasma sodium–causes, consequences, and correction. N. Engl. J. Med. 372, 55–65 (2015).
Kampmeyer, D., Lehnert, H., Moenig, H., Haas, C. S. & Harbeck, B. A strong need for improving the education of physicians on glucocorticoid replacement treatment in adrenal insufficiency: an interdisciplinary and multicentre evaluation. Eur. J. Intern. Med. 33, e13–e15 (2016).
Harbeck, B. et al. Glucocorticoid replacement therapy in adrenal insufficiency–a challenge to physicians? Endocr. J. 62, 463–468 (2015).
Hahner, S., Burger-Stritt, S. & Allolio, B. Subcutaneous hydrocortisone administration for emergency use in adrenal insufficiency. Eur. J. Endocrinol. 169, 147–154 (2013).
Burger-Stritt, S., Bachmann, L., Kurlbaum, M. & Hahner, S. Emergency treatment of adrenal crisis with prednisone suppositories: a bioequivalence study in female patients with Addison’s disease. Endocr. Connect. 8, 425–434 (2019).
Andela, C. D. et al. Quality of life in patients with adrenal insufficiency correlates stronger with hydrocortisone dosage, than with long-term systemic cortisol levels. Psychoneuroendocrinology 72, 80–86 (2016).
Lovas, K., Loge, J. H. & Husebye, E. S. Subjective health status in Norwegian patients with Addison’s disease. Clin. Endocrinol. 56, 581–588 (2002). This is the first systematic assessment of subjective health status in a large cohort of patients with primary adrenal insufficiency.
Forss, M., Batcheller, G., Skrtic, S. & Johannsson, G. Current practice of glucocorticoid replacement therapy and patient-perceived health outcomes in adrenal insufficiency – a worldwide patient survey. BMC Endocr. Disord. 12, 8 (2012).
Kluger, N. et al. Impaired health-related quality of life in Addison’s disease–impact of replacement therapy, comorbidities and socio-economic factors. Clin. Endocrinol. 81, 511–518 (2014).
Tiemensma, J. et al. Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: cross-sectional study and review of the literature. Eur. J. Endocrinol. 171, 171–182 (2014).
Ragnarsson, O. et al. The relationship between glucocorticoid replacement and quality of life in 2737 hypopituitary patients. Eur. J. Endocrinol. 171, 571–579 (2014).
Oksnes, M. et al. Quality of life in European patients with Addison’s disease: validity of the disease-specific questionnaire AddiQoL. J. Clin. Endocrinol. Metab. 97, 568–576 (2012).
Burger-Stritt, S., Pulzer, A. & Hahner, S. Quality of life and life expectancy in patients with adrenal insufficiency: what is true and what is urban myth? Front. Horm. Res. 46, 171–183 (2016).
Bleicken, B. et al. Impaired subjective health status in chronic adrenal insufficiency: impact of different glucocorticoid replacement regimens. Eur. J. Endocrinol. 159, 811–817 (2008).
Bleicken, B. et al. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin. Endocrinol. 72, 297–304 (2010).
Oksnes, M. et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of Addison’s disease: a randomized clinical trial. J. Clin. Endocrinol. Metab. 99, 1665–1674 (2014). Randomized clinical trial comparing continuous subcutaneous hydrocortisone infusion with conventional oral hydrocortisone in 33 patients with Addison disease showing more improvement in some quality of life parameters under more physiological cortisol profiles.
Nilsson, A. G. et al. Long-term safety of once-daily, dual-release hydrocortisone in patients with adrenal insufficiency: a phase 3b, open-label, extension study. Eur. J. Endocrinol. 176, 715–725 (2017).
Bancos, I. et al. Primary adrenal insufficiency is associated with impaired natural killer cell function: a potential link to increased mortality. Eur. J. Endocrinol. 176, 471–480 (2017). Analysis of 42 patients with primary AI showing normal neutrophil function but significantly decreased cytotoxicity of natural killer cells which potentially compromises antiviral immune defence.
Smans, L. C., Souverein, P. C., Leufkens, H. G., Hoepelman, A. I. & Zelissen, P. M. Increased use of antimicrobial agents and hospital admission for infections in patients with primary adrenal insufficiency: a cohort study. Eur. J. Endocrinol. 168, 609–614 (2013).
Tresoldi, A. S. et al. Increased infection risk in Addison’s disease and congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 105, 418–429 (2020).
Gan, E. H. & Pearce, S. H. Management of endocrine disease: regenerative therapies in autoimmune Addison’s disease. Eur. J. Endocrinol. 176, R123–R135 (2017).
Balyura, M. et al. Transplantation of bovine adrenocortical cells encapsulated in alginate. Proc. Natl Acad. Sci. USA 112, 2527–2532 (2015).
Gan, E. H. et al. Residual adrenal function in autoimmune Addison’s disease: improvement after tetracosactide (ACTH1-24) treatment. J. Clin. Endocrinol. Metab. 99, 111–118 (2014).
Pearce, S. H. et al. Adrenal steroidogenesis after B lymphocyte depletion therapy in new-onset Addison’s disease. J. Clin. Endocrinol. Metab. 97, E1927–E1932 (2012).
Napier, C. et al. Residual adrenal function in autoimmune Addison’s disease – effect of dual therapy with rituximab and depot tetracosactide. J. Clin. Endocrinol. Metab. 105, e1250–e1259 (2020).
Hahner, S. et al. Timelines in the management of adrenal crisis – targets, limits and reality. Clin. Endocrinol. 82, 497–502 (2015).
Burger-Stritt, S. et al. Management of adrenal emergencies in educated patients with adrenal insufficiency–a prospective study. Clin. Endocrinol. 89, 22–29 (2018).
Repping-Wuts, H. J., Stikkelbroeck, N. M., Noordzij, A., Kerstens, M. & Hermus, A. R. A glucocorticoid education group meeting: an effective strategy for improving self-management to prevent adrenal crisis. Eur. J. Endocrinol. 169, 17–22 (2013).
Burger-Stritt, S. et al. Standardised patient education in adrenal insufficiency – a prospective multi-centre evaluation. Eur. J. Endocrinol. 183, 119–127 (2020). This longitudinal, prospective, questionnaire-based, multicentre study included 526 patients with AI and proved that group education of patients with chronic AI represents a helpful tool for the guidance of patients, their self-assurance and their knowledge on prevention of adrenal crises.
Quinkler, M., Hahner, S., Johannsson, G. & Stewart, P. M. Saving lives of patients with adrenal insufficiency: a pan-European initiative? Clin. Endocrinol. 80, 319–321 (2014).
Bornstein, S. R., Bornstein, T. D. & Andoniadou, C. L. Novel medications inducing adrenal insufficiency. Nat. Rev. Endocrinol. 15, 561–562 (2019).
Nicolaides, N. C., Chrousos, G. P. & Charmandari, E. in Endotext (eds Feingold, K. R. et al) (MDText.com, Inc, 2000).
Mah, P. M. et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin. Endocrinol. 61, 367–375 (2004).
Bratland, E. & Husebye, E. S. Cellular immunity and immunopathology in autoimmune Addison’s disease. Mol. Cell Endocrinol. 336, 180–190 (2011).
Rege, J., Turcu, A. F., Else, T., Auchus, R. J. & Rainey, W. E. Steroid biomarkers in human adrenal disease. J. Steroid Biochem. Mol. Biol. 190, 273–280 (2019).
Mannstadt, M. et al. Hypoparathyroidism. Nat. Rev. Dis. Prim. 3, 17055 (2017).
Lebbe, M. & Arlt, W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin. Endocrinol. 78, 497–502 (2013).
Erichsen, M. M., Husebye, E. S., Michelsen, T. M., Dahl, A. A. & Lovas, K. Sexuality and fertility in women with Addison’s disease. J. Clin. Endocrinol. Metab. 95, 4354–4360 (2010).
Remde, H., Zopf, K., Schwander, J. & Quinkler, M. Fertility and pregnancy in primary adrenal insufficiency in Germany. Horm. Metab. Res. 48, 306–311 (2016).
Bjornsdottir, S. et al. Addison’s disease in women is a risk factor for an adverse pregnancy outcome. J. Clin. Endocrinol. Metab. 95, 5249–5257 (2010).
Schneiderman, M., Czuzoj-Shulman, N., Spence, A. R. & Abenhaim, H. A. Maternal and neonatal outcomes of pregnancies in women with Addison’s disease: a population-based cohort study on 7.7 million births. BJOG 124, 1772–1779 (2017).
Bothou, C. et al. Current management and outcome of pregnancies in women with adrenal insufficiency: experience from a multicenter survey. J. Clin. Endocrinol. Metab. 105, e2853–e2863 (2020). Large retrospective multicentre analysis of the course of pregnancy in women with adrenal insufficiency.
Hirschberg, A. L. et al. Reproductive and perinatal outcomes in women with congenital adrenal hyperplasia: a population-based cohort study. J. Clin. Endocrinol. Metab. 106, e957–e965 (2021).
Quinkler, M. et al. Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur. J. Endocrinol. 146, 789–799 (2002).
Quinkler, M., Meyer, B., Oelkers, W. & Diederich, S. Renal inactivation, mineralocorticoid generation, and 11β-hydroxysteroid dehydrogenase inhibition ameliorate the antimineralocorticoid effect of progesterone in vivo. J. Clin. Endocrinol. Metab. 88, 3767–3772 (2003).
Cosimo, C. & Franco, C. Addison’s disease and pregnancy: case report. J. Prenat. Med. 3, 53–54 (2009).
Oelkers, W. K. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids 61, 166–171 (1996).
Stirrat, L. I. et al. Transfer and metabolism of cortisol by the isolated perfused human placenta. J. Clin. Endocrinol. Metab. 103, 640–648 (2018).
Coursin, D. B. & Wood, K. E. Corticosteroid supplementation for adrenal insufficiency. JAMA 287, 236–240 (2002).
Li, D. et al. Determinants of self-reported health outcomes in adrenal insufficiency: a multi-site survey study. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaa668 (2020).
Fleming, L., Knafl, K., Knafl, G. & Van Riper, M. Parental management of adrenal crisis in children with congenital adrenal hyperplasia. J. Spec. Pediatr. Nurs. 22, e12190 (2017).
Kienitz, T., Hahner, S., Burger-Stritt, S. & Quinkler, M. Therapeutic patient education for adrenal insufficiency under COVID-19 pandemic conditions. Exp. Clin. Endocrinol. Diabetes https://doi.org/10.1055/a-1217-7208 (2020).
Acknowledgements
S.H. is supported by the Deutsche Forschungsgemeinschaft (DFG) (within the CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” and HA 6931/3-1). W.A. is supported by the Medical Research Council UK (programme grant G0900567). I.B. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under award K23DK121888. The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health, USA. E.S.H. is supported by K.G. Jebsen Center of Autoimmune Disorders, The Novonordisk Foundation, The Norwegian Research Council. The authors thank S. Aslaksen for her input in creating the figure on mechanisms underlying Addison disease.
Author information
Authors and Affiliations
Contributions
Introduction (S.H. and M.Q.); Epidemiology (I.B. and E.S.H.); Mechanisms/pathophysiology (S.H., E.S.H., R.J.R. and M.Q.); Diagnosis, screening and prevention (W.A. and M.Q.); Management (W.A., S.H., M.Q., R.J.R., S.B.-S. and D.J.T.); Quality of life (S.H.); Outlook (S.H., M.Q. and R.J.R.); Overview of Primer (S.H.).
Corresponding author
Ethics declarations
Competing interests
R.J.R. is a Director of Diurnal group PLC. W.A. served as consultant and clinical investigator for Diurnal Ltd. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks L. Chan, G. Johannsson, L. Nieman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Glucocorticoids
-
A class of corticosteroids that are essential mediators of the stress system and major players in the stress response, that regulate resting homeostasis (metabolism of carbohydrates, proteins and fats) and modulate the immune response.
- Mineralocorticoids
-
A class of corticosteroids involved in the maintenance of the salt and water balance in the body.
- Immune checkpoint inhibitors
-
Drugs typically used in oncology that stimulate antitumour immune response by blocking checkpoint proteins and promoting immune-mediated elimination of tumour cells.
- 21-Hydroxylase
-
An enzyme involved in the biosynthesis of cortisol and aldosterone.
- Autoimmune polyglandular syndromes
-
Also known as polyglandular autoimmune syndromes or autoimmune polyendocrine syndromes. These syndromes comprise a heterogeneous group of rare immune-mediated endocrinopathies affecting more than one endocrine organ. Non-endocrine organs may also be involved.
- Hypopituitarism
-
Dysfunction of the pituitary with impaired secretion or a lack of secretion of one or more pituitary hormones.
- Macula densa cells
-
Specialized cells lining the wall of kidney tubules that detect changes in distal tubular fluid composition and transmit signals to the glomerular vascular elements.
- Juxtaglomerular cells
-
Specialized cells in the kidney that synthesize, store and secrete the enzyme renin.
- Salt-wasting syndrome
-
A more severe form of congenital adrenal hyperplasia characterized by insufficient production and action of aldosterone that lead to renal sodium loss and consequent sodium depletion of the body. If undiagnosed, dehydration, hypotension, failure to thrive, hyponatraemia and hyperkalaemia occur within days of birth.
- Virilization
-
A condition in which a female develops masculine characteristics such as excess facial and body hair, acne, increased muscle mass and baldness. Virilization is caused by excess production of endogenous androgens from the adrenals or the ovaries or from exogenous androgen administration.
- Precocious pseudopuberty
-
Partial pubertal development that results from premature appearance of secondary sexual characteristics in prepubertal boys and girls owing to excess production of sex steroids.
- Leydig cells
-
Steroidogenic cells in the testes that produce testosterone upon stimulation by pituitary luteinizing hormone.
- Spastic paraparesis
-
A group of rare hereditary disorders that cause gradual weakness with muscle spasms.
- Corticotroph
-
Cells in the anterior pituitary that produce pro-opiomelanocortin, which undergoes cleavage to form adrenocorticotropic hormone (ACTH) in response to stimulation by hypothalamic corticotropin-releasing hormone.
- Adrenarche
-
Maturation of the zona reticularis of the adrenal resulting in increased production of adrenal androgens.
- ACTH 1–24 stimulation test
-
Also known as the Synacthen test or corticotropin-stimulation test. This test is commonly used for the assessment of adrenal cortisol production. Cortisol serum levels are measured before and 30–60 min after supraphysiological stimulation with synthetic 1–24 ACTH.
Rights and permissions
About this article
Cite this article
Hahner, S., Ross, R.J., Arlt, W. et al. Adrenal insufficiency. Nat Rev Dis Primers 7, 19 (2021). https://doi.org/10.1038/s41572-021-00252-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00252-7
This article is cited by
-
Iatrogenic adrenal insufficiency in adults
Nature Reviews Endocrinology (2024)
-
Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in a patient with systemic lupus erythematosus and antiphospholipid syndrome: case presentation and review of the literature
Hormones (2023)
-
Osteoporosis in Adrenal Insufficiency: Could Metformin be Protective?
Indian Journal of Clinical Biochemistry (2023)
-
Adrenal insufficiency in pregnancy: Physiology, diagnosis, management and areas for future research
Reviews in Endocrine and Metabolic Disorders (2023)
-
Adrenal crises in adolescents and young adults
Endocrine (2022)