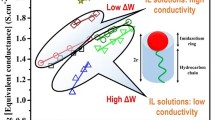
Abstract—Physicochemical properties of aqueous solutions of a number of proton ionic liquids (electrical conductivity, dissociation parameters, pH, and concentration functions) are determined using specially designed equipment and the techniques of computer resistometry. The correlations between the physicochemical properties of water–organic mixtures and a possible structure of the organic phase are established.

















Similar content being viewed by others
REFERENCES
Koshel’, N.D., Magdych, E.A., and Akimov, A.M., Regeneration of an ion exchanger in an electric field in an ion exchange column, Vopr. Khim. Khim. Tekhnol., 2010, vol. 5, p. 137.
Newman, J.S., Electrochemical System, Englewood Cliffs, NJ: Prentice-Hall, 1973.
Zoski, C.G., Handbook of Electrochemistry, Amsterdam: Elsevier, 2007.
Demirbas, A., Pehlivan, E., Gode, F., Altun, T., et al., Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin, J. Colloid Interface Sci., 2005, vol. 282, no. 1, p. 20.
Pehlivan, E. and Altun, T., Comparison of adsorption capacity of young brown coals and humic acids prepared from different coal mines in Anatolia, J. Hazard. Mater., 2006, vol. 134, nos. 1–3, p. 149.
Sverdlikovśka, O.S., Perspective of ionics liquids on bisquaternary salts of ammonium of derivates morpholyne with anion of thetrafluorineborate, Eur. Appl. Sci., 2014, vol. 7, p. 73.
Sun, J., Murthy Konda, N.V.S.N., Parthasarathi, R., Dutta, T., et al., One-pot integrated biofuel production using low-cost biocompatible protic ionic liquids, Green Chem., 2017, vol. 13. https://doi.org/10.1039/C7GC01179B
Washburn, E.W., International Critical Tables of Numerical Data, Physics, Chemistry and Technology, New York: US Natl. Res. Conc., 2003, no. 6.
McCleskey, B.R., Electrical conductivity of electrolytes found in natural waters from 5 to 90°C, J. Chem. Eng. Data, 2011, vol. 56, no. 2, p. 317.
Hunt, R.C., How to Increase the Accuracy of Solution Conductivity Measurements, Santa Ana, CA: Sensor Dev., 1995. http://www.aitsouthwest.com.
Shaplov, A.S., Lozinskaya, E.I., Ponkratov, D.O., Malyshkina, I.A., et al., Bis(trifluoromethylsulfonyl) amide based “polymeric ionic liquids”: Synthesis, purification and peculiarities of structure–properties relationships, Electrochim. Acta, 2011, vol. 57, p. 74.
Shaposhnik, V.A., Reshetnikova, A.K., Zolotareva, R.I., and Isaev, N.I., Water demineralization by electrodialysis using intermembrane filling of ion exchanger sections, Zh. Prikl. Khim., 1973, vol. 46, no. 12, p. 2659.
Özgür, A., Ümran, Y., Nalan, K., and Mithat, Y., Various applications of electrodeionization (EDI) method for water treatment—A short review, Desalination, 2014, vol. 342, p. 16.
Smara, A., Delimi, R., Chai-Net, E., and Sandeaux, J., Removal of heavy metals from diluted mixtures by a hybrid ion-exchange/electrodialysis process, Sep. Purif. Technol., 2007, vol. 57, no. 1, p. 103.
Davies, P.A. and Orfi, J., Self-powered desalination of geothermal saline groundwater: Technical feasibility, Water, 2014, vol. 6, no. 11, p. 3409. https://doi.org/10.3390/w6113409
Alvarado, L. and Chen, A., Electrodeionization: principles, strategies and applications, Electrochim. Acta, 2014, vol. 132, p. 583.
Dzyazko, Yu.S. and Belyakov, V.N., Purification of a diluted nickel solution containing nickel by a process combining ion exchange and electrodialysis, Desalination, 2004, vol. 162, p. 179.
Shvedene, N.V., Chernyshov, D.V., and Pletnev, I.V., Ionic liquids in electrochemical sensors, Russ. J. Gen. Chem., 2008, vol. 78, no. 12, p. 2507.
Spravochnik khimika. Tom 3. Khimicheskoe ravnovesie i kinetika svoistva rastvorov. Elektrodnye protsessy (Handbook of Chemist, Vol. 3: Chemical Balance and Kinetics of Solution Properties. Electrode Processes), 2nd ed., Moscow: Khimiya, 19651965.
Koshel’, N.D. and Kostyrya, M.V., Conductance-measuring method for quantitative analysis of two-component electrolyte solutions, Surf. Eng. Appl. Electrochem., 2018, vol. 54, no. 1, p. 103.
Koshel, N.D., Smyrnova, O.V., and Kostyrya, M.V., The study of ion exchange kinetics on the anionite in weak electric fields, Proc. 8th International Conf. “Materials Science & Condensed Matter Physics,” September 12–16, 2016, Abstracts of Papers, Chisinau, 2016, p. 340.
Koshel, N.D., Smirnova, E.V., Burtovaya, V.P., and Koshel’, S.A., The exchange properties of the AN-2FN anionite by the computer resistometry, Vopr. Khim. Khim. Tekhnol., 2018, no. 5, pp. 23–30.
Greaves, T.L. and Drummond, C.J., Protic ionic liquids: properties and applications, Chem. Rev., 2008, vol. 108, no. 1, p. 206.
Drozd, G.I., Fosfaty organicheskie. Khimicheskaya entsiklopediya (Organic Phosphates. Chemical Encyclopedia), Moscow: Bol’shaya Rossiiskaya Entsiklopediya, 1998, vol. 5, pp. 131−132. https://ru.wikipedia.org/wiki/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Myshkina
About this article
Cite this article
Koshel, N.D., Koshel, S.A., Sverdlikovskaya, O.S. et al. Equilibrium States in Aqueous Solutions of Some Ionic Liquids. Surf. Engin. Appl.Electrochem. 57, 88–100 (2021). https://doi.org/10.3103/S1068375521010063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375521010063