Abstract
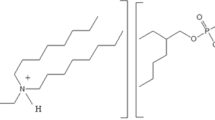
This study addresses the liquid-liquid extraction behavior of phosphorus from a sulfuric acid solution using benzyl dimethyl amine (BDMA) in kerosene. The extraction equilibria investigated with varied BDMA concentrations could reveal the formation of \(\overline {3[{\rm<Emphasis Type="Bold">MA</Emphasis>}] \cdot [{{\rm{H}}_3}{\rm{P}}{{\rm{O}}_4}]} \) complex in the organic phase. The thermodynamic properties determined at various temperatures indicated that the process was exothermic with a calculated enthalpy (ΔH⊖) of −24.0 kJ·mol−1. The organic-to-aqueous phase (O/A) volume ratio was varied to elucidate the quantitative extraction of phosphorus. The McCabe-Thiele diagram plotted for the extraction isotherm was validated for the requirement of three counter-current stages in the extraction at an O/A volume ratio of 2.0/3.5. The back-extraction of phosphorus from the loaded organic phase was quantitatively achieved by contacting 4.0 mol·L−1 H2SO4 solution in three stages of counter-current contact at an O/A volume ratio of 3/2. This study can be applied to remove phosphorus from the sulfuric acid leach solutions of monazite processing, and many other solutions.
Similar content being viewed by others
References
E.H. Borai, M.S. Abd El-Ghany, I.M. Ahmed, M.M. Hamed, A.M. Shahr El-Din, and H.F. Aly, Modified acidic leaching for selective separation of thorium phosphate and rare earth concentrates from Egyptian crude monazite, Int. J. Miner. Process., 149(2016), p. 34.
M.S. Alshammari, I.M. Ahmed, A.A. Nayl, H.F. Aly, G.G. Mohamed, and S.A.R. Mostafa, An assessment for the recovery of lanthanides and P2O5 from phosphate rocks, Adv. Environ. Biol., 10(2016), No. 9, p. 49.
Y. Kanazawa and M. Kamitani, Rare earth minerals and resources in the world, J. Alloys Compd., 408–412(2006), p. 1339.
Y.A. El-Nadi, J.A. Daoud, and H.F. Aly, Modified leaching and extraction of uranium from hydrous oxide cake of Egyptian monazite, Int. J. Miner. Process., 76(2005), No. 1–2, p. 101.
L. Zhang, J. Chen, W.Q. Jin, Y.F. Deng, J. Tian, and Y. Zhang, Extraction mechanism of cerium (IV) in H2SO4/H3PO4 system using bifunctional ionic liquid extractants, J. Rare Earths, 31(2013), No. 12, p. 1195.
D. Zou, J. Chen, and D.Q. Li, Separation chemistry and clean technique of cerium (IV): A review, J. Rare Earths, 32(2014), No. 8, p. 681.
Environmental Agency, Aquatic eutrophication in England and Wales: Proposed management strategy, [in] Environmental Issue Series, Environmental Agency, Bristol, 2003.
S. Burk, A.L. Heathwaite and N. Preedy, Transfer of phosphorus to surface waters: Eutrophication, [in] Phosphorusin Environmental Technologies Principles and Applications, IWA Publishing, London, 2004, p. 120.
J.Q. Jiang and Q. Mwabonje, Phosphorus recovery by liquid-liquid extraction, Sep. Sci. Technol., 44(2009), No. 13, p. 3258.
M. Yang, Q.S. Zhu, C.L. Fan, Z.H. Xie, and H.Z. Li, Roasting-induced phase change and its influence on phosphorus removal through acid leaching for high-phosphorus iron ore, Int. J. Miner. Metall. Mater., 22(2015), No. 4, p. 346.
J.Q. Jiang and N.J.D. Graham, Pre-polymerised inorganic coagulants and phosphorus removal by coagulation — A review, Water SA, 24(1998), No. 3, p. 237.
U. Berg and C. Schaum, Recovery of phosphorus from sewage sludge and sludge ashes — Application in Germany and Northern Europe, [in] Proceedings of the I. National Sludge Symposium, Izmirs, 2005, p. 87.
G. Tchobanoglous and F.L. Burton, Wastewater Engineering—Treatment, Disposal and Reuse, 4th ed., Metcalf and Eddy, Inc., eds., McGraw Hill, New York, 2003.
R.R. Srivastava, S. Ilyas, H. Kim, S. Choi, H.B. Trinh, M.A. Ghauri, and N. Ilyas, Biotechnological recycling of critical metals from waste printed circuit boards, J. Chem. Technol. Biotechnol., 95(2020), No. 11, p. 2796.
M.A. Muhsan, S. Ilyas, H.A. Cheema, S. Masud, and N. Shabbir, Recovery of nitric acid from effluent streams using solvent extraction with TBP: A comparative study in absence and presence of metal nitrates, Sep. Purif. Technol., 186(2017), p. 90.
G.M. Ritcey and A.W. Ashbrook, Solvent Extraction. Principles and Applications to Process Metallurgy, Part I, Elsevier, Amsterdam, 1984.
R.X. Mu, J. Chen, D. Zou, K. Li, and D.Q. Li, Liquid-liquid extraction and recovery of Cerium (IV) and Phosphorus from sulfuric acid solution using Cyanex 923, Sep. Purif. Technol., 209(2019), p. 351.
O.N. Mwabonje and J.L. Jiang, A trial of using solvent extraction for phosphorus recovery, J. Water Res. Prot., 2(2010), No. 9, p. 830.
J.A. Rard and T.J. Wolery, The standard chemical-thermodynamic properties of phosphorus and some of its key compounds and aqueous species: An evaluation of differences between the previous recommendations of NBS/NIST and CODATA, J. Solution Chem., 36(2007), No. 12, p. 1585.
D.D. Wagman, W.H. Evans, V.B. Parker, I. Halow, S.M. Bailey, and R.H. Schumm, Selected Values of Chemical Thermodynamic Properties, Tables for the First Thirty-Four Elements in the Standard Order of Arrangement, NBS Technical Note 270–3, U.S. Government Printing Office, Washington, 1968.
M. Cox, Solvent extraction in hydrometallurgy, [in] Solvent Extraction Principles and Practice, Revised and Expanded, CRC Press, New York, 2004, p. 466.
R.R. Srivastava, S. Ilyas, H. Kim, N.L.M. Tri, N. Hassan, M. Mudassir, and N. Talib, Liquid-liquid extraction and reductive stripping of chromium to valorize industrial effluent, JOM, 72(2020), No. 2, p. 839.
Y. Marcus, Solvent extraction of inorganic species, Chem. Rev., 63(1963), No. 2, p. 139.
V.S. Kislik, Solvent Extraction: Classical and Novel Approaches, 1st ed., Elsevier, Amsterdam, 2011.
D.L. Pavia, G.M. Lampman, G.S. Kriz, and J.R. Vyvyan, Introduction of Spectroscopy, Brooks/Cole Cengage Learning, Belmont, 2001.
Acknowledgements
This work was supported by the Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Grant No. 2019H1D3A2A02101993). The author Sadia Ilyas is grateful to NRF for presenting the Brain Pool Scientists award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilyas, S., Srivastava, R.R. & Kim, H. Liquid-liquid extraction of phosphorus from sulfuric acid solution using benzyl dimethyl amine. Int J Miner Metall Mater 28, 367–372 (2021). https://doi.org/10.1007/s12613-020-2151-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-020-2151-8