Abstract
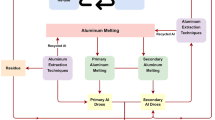
As a by-product of the steelmaking industry rich in iron and zinc, electric arc furnace dust (EAFD) landfill disposal is restricted in many countries due to the metal lixiviation risks. Several approaches have been examined to recycle or reuse EAFD. Hydrometallurgical methods mainly include the preparation of pregnant acid leached solution (PLS) using inorganic acids followed by selective metal recovery using different methods. We herein proposed a hydrometallurgical process initiated by acid leaching and finalized by thermal treatment to produce nano-ZnO from EAFD. At the condition of this study, only 67% of zinc was leached by sulfuric acid from EAFD into PLS while 98% of surplus leached iron was removed through precipitation by sodium hydroxide. Additional purification of iron removed PLS was accomplished using di-2 ethylhexyl phosphoric acid to extract Zn+2. The addition of ammonium hydroxide to purified PLS provided (Zn(OH)2)3(ZnSO4)(H2O)5 as the final precursor for nano-ZnO that ultimately transformed to nano-ZnO (60 ± 6 nm particle size) at 850 °C. The achieved results were scrutinized through various tests and analyses including TEM, XRD, SEM, EDS, FTIR, and TGA.
Graphical Abstract











Similar content being viewed by others
References
Baba AA, Adekola FA (2011) Beneficiation of a Nigerian sphalerite mineral: solvent extraction of zinc by Cyanex® 272 in hydrochloric acid. Hydrometallurgy 109:187–193
Bakhtiari F, Darezereshki E (2011) One-step synthesis of tenorite (CuO) nano-particles from Cu4 (SO4)(OH) 6 by direct thermal-decomposition method. Mater Lett 65:171–174
Brunelli K, Dabalà M (2015) Ultrasound effects on zinc recovery from EAF dust by sulfuric acid leaching. Int J Miner Metall Mater 22:353–362
Cashman JB (1998) Hydrometallurgical processing of flue dust. In: Google Patents. Patent No. US005709730A: 1-9
Darezereshki E (2010) Synthesis of maghemite (γ-Fe2O3) nanoparticles by wet chemical method at room temperature. Mater Lett 64:1471–1472
Darezereshki E, Alizadeh M, Bakhtiari F, Schaffie M, Ranjbar M (2011) A novel thermal decomposition method for the synthesis of ZnO nanoparticles from low concentration ZnSO4 solutions. Appl Clay Sci 54:107–111
Darezereshki E, Bakhtiari F, Alizadeh M, Ranjbar M (2012) Direct thermal decomposition synthesis and characterization of hematite (α-Fe2O3) nanoparticles. Mater Sci Semicond Process 15:91–97
Darezereshki E, Ranjbar M, Bakhtiari F (2010) One-step synthesis of maghemite (γ-Fe2O3) nano-particles by wet chemical method. J Alloys Compd 502:257–260
Geetha D, Thilagavathi T (2010) Hydrothermal synthesis of nano ZnO structures from CTAB. Dig J Nanomater Biostruct 5:297–301
Halli P, Hamuyuni J, Leikola M, Lundström M (2018) Developing a sustainable solution for recycling electric arc furnace dust via organic acid leaching. Miner Eng 124:1–9
Halli P, Hamuyuni J, Revitzer H, Lundström M (2017) Selection of leaching media for metal dissolution from electric arc furnace dust. J Clean Prod 164:265–276
Hammad TM, Salem JK, Harrison RG (2009) Synthesis, characterization, and optical properties of Y-doped ZnO nanoparticles. Nano 4:225–232
Hasnidawani JN, Azlina HN, Norita H, Bonnia NN, Ratim S, Ali ES (2016) Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem 19:211–216
Havlik T, Turzakova M, Stopic S, Friedrich B (2005) Atmospheric leaching of EAF dust with diluted sulphuric acid. Hydrometallurgy 77:41–50
Jha MK, Kumar V, Singh RJ (2001) Review of hydrometallurgical recovery of zinc from industrial wastes. Resour Conserv Recycl 33:1–22
Koohestani B, Darban AK, Darezereshki E, Mokhtari P, Yilmaz E, Yilmaz E (2018) The influence of sodium and sulfate ions on total solidification and encapsulation potential of iron-rich acid mine drainage in silica gel. J Environ Chem Eng 6:3520–3527
Koohestani B, Darban AK, Mokhtari P, Darezereshki E, Yilmaz E, Yilmaz E (2020) Influence of hydrofluoric acid leaching and roasting on mineralogical phase transformation of pyrite in sulfidic mine tailings. Minerals 10:513
Kurama H, Çatalsarik T (2000) Removal of zinc cyanide from a leach solution by an anionic ion-exchange resin. Desalination 129:1–6
Leclerc N, Meux E, Lecuire J-M (2002) Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitrilotriacetate anion and hexahydrated ferric chloride. J Hazard Mater 91:257–270
Li X, Monnens W, Zheng L, Fransaer J, Binnemans K (2020) Solvometallurgical process for extraction of copper from chalcopyrite and other sulfidic ore minerals. Green Chem 22:417–426
Liang JZ (2020) Melt Elongation Flow Behavior of Low-Density Polyethylene Composites Filled with Nanoscale Zinc Oxide. J Test Eval 48:4551–4562
Lin X, Peng Z, Yan J, Li Z, Hwang J-Y, Zhang Y, Li G, Jiang T (2017) Pyrometallurgical recycling of electric arc furnace dust. J Clean Prod 149:1079–1100
Lis T, Nowacki K, Żelichowska M, Kania H (2015) Innovation in metallurgical waste management. Metalurgija 54:283–285
Machado JGMS, Brehm FA, Moraes CAM, Santos CAD, Vilela ACF, Da Cunha JBM (2006) Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J Hazard Mater 136:953–960
Madhi A, Hadavand BS (2020) Eco-friendly castor oil-based UV-curable urethane acrylate zinc oxide nanocomposites: synthesis and viscoelastic behavior. J Compos Mater 54:101–110
Maweja K, Mukongo T, Mbaya RK, Mochubele EA (2010) Effect of annealing treatment on the crystallisation and leaching of dumped base metal smelter slags. J Hazard Mater 183:294–300
Montenegro V, Oustadakis P, Tsakiridis PE, Agatzini-Leonardou S (2013) Hydrometallurgical treatment of steelmaking electric arc furnace dusts (EAFD). Metall Mater Trans B 44:1058–1069
Muruganandham M, Chen IS, Wu JJ (2009) Effect of temperature on the formation of macroporous ZnO bundles and its application in photocatalysis. J Hazard Mater 172:700–706
Niubó M, Fernández AI, Chimenos JM, Haurie L (2009) A possible recycling method for high grade steels EAFD in polymer composites. J Hazard Mater 171:1139–1144
Omran M, Fabritius T, Heikkinen E-P (2019) Selective Zinc Removal from Electric Arc Furnace (EAF) Dust by Using Microwave Heating. J Sustain Metall 5:331–340
Oustadakis P, Tsakiridis PE, Katsiapi A, Agatzini-Leonardou S (2010) Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterization and leaching by diluted sulphuric acid. J Hazard Mater 179:1–7
Pickles CA (2010) Thermodynamic modelling of the formation of zinc–manganese ferrite spinel in electric arc furnace dust. J Hazard Mater 179:309–317
Ruiz O, Clemente C, Alonso M, Alguacil FJ (2007) Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J Hazard Mater 141:33–36
Spanhel L (2006) Colloidal ZnO nanostructures and functional coatings: a survey. J Sol-Gel Sci Technol 39:7–24
Tsakiridis PE, Oustadakis P, Katsiapi A, Agatzini-Leonardou S (2010) Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part II: Downstream processing and zinc recovery by electrowinning. J Hazard Mater 179:8–14
Vakylabad AB (2011) A comparison of bioleaching ability of mesophilic and moderately thermophilic culture on copper bioleaching from flotation concentrate and smelter dust. Int J Miner Process 101:94–99
Vakylabad AB, Schaffie M, Naseri A, Ranjbar M, Manafi Z (2016) A procedure for processing of pregnant leach solution (PLS) produced from a chalcopyrite-ore bio-heap: CuO Nano-powder fabrication. Hydrometallurgy 163:24–32
Wang H-g, Yang L, Gao J-m, Zhang M, Guo M (2016) A novel hydrothermal method for zinc extraction and separation from zinc ferrite and electric arc furnace dust. Int J Miner Metall Mater 23:146–155
Weiβ Th, Xalter R (2014) Nano silver-zinc oxide composition. In: Google Patents. Patent No. US008951543B2: 1-11
Woolard CD, Williams DC, Van Rooyen JL, Garde K, Bosch RM, Strydom SHJ (2013) Non-polar capped nano transition metal oxides and sulfides. In: Google Patents. Patent No. US20100305332A1: 1-9.
Xanthopoulos P, Agatzini-Leonardou S, Oustadakis P, Tsakiridis PE (2017) Zinc recovery from purified electric arc furnace dust leach liquors by chemical precipitation. J Environ Chem Eng 5:3550–3559
Ye Q, Peng Z, Li G, Lee J, Liu Y, Liu M, Wang L, Rao M, Zhang Y, Jiang T (2019) Microwave-assisted reduction of electric arc furnace dust with biochar: an examination of transition of heating mechanism. ACS Sustain Chem Eng 7:9515–9524
Yu B-S, Wang Y-R, Chang T-C (2011) Hydrothermal treatment of electric arc furnace dust. J Hazard Mater 190:397–402
Zhan L, Li O, Zhenming X (2020) Preparing nano-zinc oxide with high-added-value from waste zinc manganese battery by vacuum evaporation and oxygen-control oxidation. J Clean Prod 251:119691
Zhang Y, Zhang Y, Zhang S, Yang G, Gao C, Zhou C, Zhang C, Zhang P (2020) One step synthesis of ZnO nanoparticles from ZDDP and its tribological properties in steel-aluminum contacts. Tribol Int 141:105890
Acknowledgements
This work was financially supported by the IRANIAN National science foundation (No. 90003855). We also want to appreciate the cooperation of Dr. Ali Khodayari from the SEM/EDS analysis unit of University of Mohaghegh Ardabili.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Darezereshki, E., Vakylabad, A.B. & Koohestani, B. A Hydrometallurgical Approach to Produce Nano-ZnO from Electrical Arc Furnace Dusts. Mining, Metallurgy & Exploration 38, 1525–1535 (2021). https://doi.org/10.1007/s42461-021-00412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00412-z