Abstract
Two semi-synthetic clay-based catalysts were prepared. These catalysts were obtained by incorporating lanthanum oxide (Cat1) and chromium oxide (Cat2). They were then tested for catalytic cracking of a heavy petroleum residue (fuel). The two formulations were carried out in the presence of silica to improve their acidity then underwent an acid activation. The catalysts obtained were characterized by various methods (XRD, FTIR, ICP-OES, SEM). The results showed that the incorporation of oxides and the addition of silica improves the structural characteristics of the final products. The support used was a kaolinite rich clay, having a specific surface area of 15.26 m2/g and acidity of 14 meq/g. These values increase, respectively, to 456.14 m2/g and 50 meq/g for Cat1 and to 475.12 m2/g and 57 meq/g for Cat2. The influence of the type of oxide incorporated, the specific surface area, the porosity and the acidity of the catalysts on their catalytic activity was studied. The nature of the oxide used proved to be decisive on the quality of the catalyst. Thus Cat1, prepared with lanthanum oxide, showed the best performance in cracking the petroleum residue achieving a conversion rate of 74.13% compared to 66.53% for cat2.
Similar content being viewed by others
Introduction
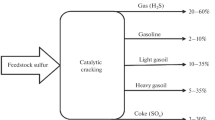
Due to its impact on the environment, the use of heavy oil residue (fuel) is strongly discouraged by government officials. Thus, the demand of fuel will suffer a sharp drop in coming years in favor of gasoline, diesel and other petroleum products, which are lighter. To remain competitive and give added value to heavy oil residues, the refineries are stepping up their research to improve the fluid catalytic cracking (FCC) process [9, 19]. The FCC process consist to convert heavy oil feeds into light products. Although this process has been used in refineries for decades, it needs to be innovate to achieve performance [2, 25]. Therefore, new catalyst development need to be investigated to improve the yield of FCC processes.
A catalyst is a substance that increases the rate of a chemical reaction, without being consumed or produced [8]. The activity and selectivity of the FCC catalyst are derived from the acidic sites and the pore structure, respectively. Catalytic catalysts are therefore porous solids with acidic properties [4, 18, 23]. Commercial FCC catalysts generally consist of two main components: the zeolite and the matrix. The zeolites used in FCC catalysts are mainly synthetic faujasite Y type zeolites and high silica Y zeolites, which are the main contributor to the catalytic activity and selectivity of the FCC catalyst [13]. The matrix is made of different constituents such as aluminosilicates and additives which are solid compounds added to improve its properties [4, 9]. Nevertheless, the high cost of these commercial catalysts could be a barrier to their use in development countries. It is urgent to develop a novel FCC catalyst with local resource [20, 26].
Recent research is directed towards the development of semi-synthetic clay-based catalysts. In this configuration, the clay acts as a matrix in which metallic oxides are incorporated. Emam [8] has shown that clay catalysts are arousing much interest for catalytic application in the petroleum refining industry. According to Bouras [6], the interest given in recent years to the study of clays by laboratories around the world is explained by their abundance in nature, the importance of their specific surface, their porosity and especially their ability to exchange interfoliar cations. Kaolin and montmorillonite are the most commonly used clays in the development of refining catalysts [20]. Murray [21] estimates that more than 200,000 tonnes of kaolin are used annually to produce petroleum cracking catalysts. This work deals with the development of new semi-synthetic catalysts based on clay and oxides, the characterization of these catalysts obtained and finally their use in fuel cracking. To our knowledge, in the literature, it has not been reported cracking of fuel oil by semi-synthetic catalysts or even by conventional catalytic catalysts. This study therefore aims to fill this void.
The objective of the current work was to prepare a FCC catalyst, and to study the catalytic properties. Two catalysts were prepared using a kaolinite rich clay as matrix. The influence of the type of oxide incorporated, the specific surface area, the porosity and the acidity of the catalysts on their catalytic activity was studied.
Materials and methods
Materials
The catalysts prepared in this study were made from Niger clay. This clay has been characterized in previous work [1]. It has a specific surface area of 15.26 m2/g and a loss on ignition of 16.1%. It is mainly composed of kaolinite (46.3%) and an interstratified smectite illite chlorite (53.7%) [1]. The choice of this clay is explained because the catalytic reactions taking place at high temperature (≥ 500 °C), the catalyst must be a refractory material, able to withstand such temperature.
Catalyst preparation
In a beaker containing 15 g of clay with a particle size less than 2 µm, 0.55 g of lanthanum (La2O3) or chromium (Cr2O3) oxide and 4 g of silica are added. After homogenization, acid activation is carried out by adding 25 mL of 10% hydrochloric acid and then the whole is left under stirring for 6 hours. The resulting mixture is dried at 105 °C for 12 hours and then calcined at 800 °C for thirty minutes. The catalyst containing lanthanum oxide is called Cat1 and the one obtained with chromium oxide is called Cat2.
Catalyst characterization
X-ray diffraction (XRD) patterns of all samples were recorded at room temperature, using a Rigaku-Miniflex II diffractometer (Japan). The incident radiation is generated by the Kα line of copper (λ = 1.5406 Å) at 30 kV and 15 mA. The analyzes were carried out in the angular interval [5–70°] in 2θ with a step of 0.02° (2θ) and a counting time of 2 s.
The infrared spectroscopic analyzes were carried out in ATR (Attenuated Total Reflectance) mode with a Fourier Bruker Alpha Transform spectrometer equipped with a diamond crystal (refractive index of the diamond 2.451). The spectra were acquired with a nominal resolution of 4 cm−1 over a wavenumber range from 400 to 4000 cm−1.
Chemical analysis of the samples was performed using a Vista Pro Varian instrument equipped with an ICP-OES (Inductively Coupled Plasma-Optical Emission Spectroscopy) plasma.
The BET isotherms were obtained using a Nova Station B sorptiometer. The absorption gas used is nitrogen and the measurements are carried out at 77.350 K. The determination of the microporous volumes and the microporous surfaces are carried out by the t-plot method.
A variable pressure scanning electron microscope (SEM) from the D.C.A.R. (SEM FEG Supra 40 VP Zeiss) of 2 nm resolution coupled to an X-ray microanalyzer (EDS) was used for the characterization of the microstructure and the surface chemical composition of the catalysts.
The total acidity of the catalysts was determined using the titration method of Boehm [5] which is used by many researchers working on adsorbents [12]. This method consists of assaying functional groups having various acidities with different bases. For the present study, the bases used are sodium hydroxide (NaOH), sodium hydrogen carbonate (NaHCO3) and sodium ethanolate (C2H5ONa). The procedure is as follows: a mass of 500 mg of catalyst is placed in Erlenmeyer flasks. Then 25 mL of the different bases, all prepared at 0.1 N, are transferred into each of the Erlenmeyer flasks containing the catalysts. The blank tests are carried out by proceeding in the same manner, with the difference that Erlenmeyer flasks do not contain a catalyst. The samples and the blanks are stirred on magnetic stirrers at 150 rpm for 72 hours at room temperature, then left to stand for 6 hours. The supernatant is filtered through a Whatman cellulose nitrate membrane (0.2 μm) then the excess basic solution is dosed back with a 0.1 N HCl solution.
Catalytic cracking test
The performance tests of the catalysts were carried out in a stainless steel reactor (Fig. 1), manufactured in the laboratory (LAPISEN). The methodology used here has been adapted to that of Houdry [14]. To carry out the cracking operation, a mass of 20 g of catalyst is placed in a reactor. Then, the reactor is inserted into an electric furnace and the temperature is raised in steps of 100 °C until reaching 500 °C. After checking the seal of the installation, a mass of 50 g of pre-heated petroleum residue is injected into the reactor. The reactor temperature is constantly checked by a probe thermometer (TYPE K/J) and maintained at 500 °C. The cracked products leave the reactor by passing through a refrigerating condenser and are recovered in the form of droplets in recipe flasks. The cracking operation is stopped when there is no more droplet drop in the receiving bottle.
The yield (Eq. 1) was calculated from the ratio between the mass of the product obtained after cracking (mo) and the initial mass introduced into the reactor (mi).
Results and discussion
XRD
The two catalysts developed (Cat1 and Cat2) were analyzed by the XRD method. The results obtained are given in Fig. 2. The XRD pattern of the clay (support) shows various clay minerals. The presence of smectite and illite is indicated by the peaks located at 6° and 9° respectively. The intense peaks at 12.5° and 25°are relative to kaolinite and the peak appearing around 26.78° is characteristic of quartz. It can also be seen that the XRD pattern of clay exhibited the crystalline features. The XRD patterns of the catalysts show the disappearance of all kaolinite peaks initially present in clay. Indeed, from 580 °C, kaolinite (Al2O3, 2SiO2, 2H2O) loses the hydroxyl OH function and it is transformed into metakaolinite (Al2O3, 2SiO2). This is confirmed by the XRD patterns of the catalysts that show a characteristic appearance of an amorphous material. The presence of metakaolinite, a refractory material, in these catalysts is an advantage for their use as cracking catalysts.
Moreover, the presence of La2SiO5 is identified at 27.84° (2θ) [7] and 34.9° (2θ) on the Cat1 spectrum [29]. This oxide (La2SiO5) results from the reaction between lanthanum oxide and silica, as predicted by Sun [29], in the following reaction (Eq. 2):
The characteristic phases of chromium oxide (Cr2O3) have been identified on this spectrum. They correspond to the peaks at 54.14°; and 64.04° (2θ) according to Karimi [17].
FTIR spectroscopy
The Fig. 3 shows the IR spectra of the clay (support) and the two catalysts produced. The spectrum of the support is characteristic of a clay. It shows the deformation vibration modes of structural hydroxyl groups between 950 and 800 cm−1. The absorption bands observed at wavenumbers 1034, 1100 cm−1 and 1159 cm−1 are characteristic of the antisymmetric modes of elongation of Si–O and Al–O bonds in aluminosilicates [10]. A comparative analysis of the spectrum of the support and those of the catalysts shows a remarkable change in structure. The difference observed could be related on the one hand to the new elements that were added in the preparation and on the other hand to the calcination that the catalysts underwent. The absorption band at 500 cm−1 is attributed to the vibration mode of the La–O bond of lanthanum oxide according to Saravani and Khajehali [28]. This peak confirms the presence of La2O3 phase in the Cat1 sample. The bands observed around 581 and 647 cm−1 are characteristic absorption bands of Cr2O3 on the Cat2 spectrum according to Nguyen et al. [22].
Catalyst acidity
The acidity of the catalysts measured by Boehm method indicates higher values of acidity for the catalysts compared to that of the support. Initially equal to 14 meq/g for the support, the acidity was evaluated at 50 meq/g for Cat 1 and 57 meq/g for Cat2. This significant increase could be justified by the presence of oxides which were added during processing. These acidity values are lightly superiors to those of the FCC zeolite catalysts (30–50 meq/g) used by Ibarra et al. [16]. This suggests that the catalysts developed here would be efficient in cracking test as industrial catalysts from the point of view of surface acidity.
Chemical analysis
The chemical analysis of catalysts are given in Table 1. The SiO2/Al2O3 ratio of the support was equal to 2.08, similar to that obtained by Gueu et al. [11]. The SiO2 content in Cat1 and Cat2 catalysts are 59.1% and 61.9% respectively. This content was initially equal to 49% in the support. The observed increase is due to the addition of silica during the preparation of the catalysts. According to Otmani [24], silica give good mechanical strength to catalysts and increase their acidic character [18] for the catalytic cracking operation. Furthermore, the SiO2 contents of the catalysts are higher than that of the commercial FCC catalyst (54.1%) used by Hussain et al. [15] for the catalytic cracking of vacuum gas oil.
Textural analysis
Specific surface
The specific surfaces of the catalysts produced were evaluated by the method of Brunauer, Emmet and Teller (B. E. T). They are 456.14 m2/g and 475.12 m2/g for the Cat1 and Cat2 respectively. The results obtained show that the surfaces have increased considerably compared to the specific surface of the clay support (15.26 m2/g). This is due to the acid activation performed during formulation. This considerable increase is also observed in the literature [3, 13] and reflects a significant development of microporosity. The values obtained in this study are better than 347 m2/g and 177 m2/g, representing the surface areas calculated by He et al. [13] and Al-Khattaf [3] respectively. In addition, Ribeiro et al. [27] found specific surface areas of 266, 276 and 422 m2/g for commercial FCC catalysts which are also lower than those of the catalysts developed here. These results once again show the good characteristics developed by the catalysts prepared in this work.
Porosity
The porosity of the catalyst is a very important factor. It is established that the diffusion of molecules from the residue to be cracked is easy when the dimensions of the pore are 2–6 times larger than those of the molecules [30]. The data (Table 2) show that the diameters and the pore volumes increased considerably after the preparation of the catalysts. The type of oxide used does not influence the pore diameter which remained similar for the two catalysts. The pore volume developed by Cat1 is significantly greater than that of Cat2.
SEM
The images obtained by scanning electron microscopy (SEM) and X-ray microanalysis of the catalysts are presented in Figs. 4 and 5.
Figures 4 and 5 show that the catalysts mostly consist of very fine particle clusters. These images show a certain homogeneity in the composition of the samples. However, the Fig. 4 shows clusters in the form of aggregates. This particularity could be justified by an incomplete grinding of the Cat1 sample during its SEM preparation. In addition, grains of quartz (SiO2) are also observed on these images. This confirms the above analyses.
The results of the X-ray microanalysis of the catalysts indicate the presence of several elements such as silicon (Si) which is the most abundant element in all samples. It’s to be note the appearance of lanthanum (La) in Fig. 4 and the appearance of the element chromium (Cr) in Fig. 5. The presence of these elements (La and Cr) confirms the incorporation of the oxides.
Catalytic cracking yield
The catalysts developed here were used in the cracking of a petroleum residue. The cracking tests take place at 500 °C with a ratio fuel/catalyst (g/g) equal to 4.5. The results recorded show a conversion rate of 74.13 and 66.53% for the Cat1 and Cat2 respectively. The acidity of the catalysts is very often considered to be the most important parameter. The greater the activity, the greater the catalytic efficiency. This assertion is not verified in this study. Indeed, despite its low acidity, Cat1 has the best yield. This would be attribute to its specific surface area and its pore volume which are high than those measured for Cat2.
Conclusion
The objective of this work was to develop clay-based catalysts to crack a heavy oil residue. XRD and infrared analyzes of the prepared catalysts confirmed the presence of lanthanum oxide and chromium oxide. This indicates that the oxides have been well incorporated showing at the same time that the method of preparation is suitable. Textural analysis showed that the incorporation of oxide, acid activation and calcination carried out during processing strongly influenced the composition and textural properties of the catalysts. The specific surfaces increase from 15.26 m2/g for the support to 456.14 and 475.12 m2/g for the Cat1 and Cat2 respectively. Pore volumes also increased from 0.18 cm3/g for the support to 0.49 cm3/g and 0.24 cm3/g for Cat1 and Cat2 respectively. Compared to the support, the acidities of the two catalysts are very higher. This was related to the addition of silica and acidic activation made during catalysts preparation. Finally, the catalytic cracking results indicated a conversion rate of 74.13 and 66.53% for Cat1 and Cat2 respectively. From this study, the use of this clay for the development of semi-synthetic catalysts would be particularly interesting to the refining industry.
References
Abdoulaye ODM, Yao BK, Ahmed AM, Adouby K, Abro DMK, Drogui P (2019) Mineralogical and morphological characterization of a clay from Niger
Akah A (2017) Application of rare earths in fluid catalytic cracking: a review. J Rare Earths 35:941–956
Al-Khattaf S (2003) The influence of alumina on the performance of FCC catalysts during hydrotreated VGO catalytic cracking. Energy Fuels 17:62–68
Avidan AA (1993) Origin development and scope of FCC catalysis in: studies in surface science and catalysis. Elsevier
Boehm HP (1966) Chemical identification of surface groups in: advances in catalysis. Elsevier
Bouras O (2003) Propriétés adsorbantes d’argiles pontées organophiles: synthèse et caractérisation (PhD Thesis). Limoges
Djoudi L (2016) Synthese et propriétés d’oxydes mixtes a base de lanthane, aluminium et Nickel (PhD Thesis). Université Mohamed Khider-Biskra
Emam EA (2013) Clays as catalysts in petroleum refining industry. ARPN J Sci Technol 3:356–375
Feng R, Qiao K, Wang Y, Yan Z (2013) Perspective on FCC catalyst in China. Appl Petrochem Res 3:63–70. https://doi.org/10.1007/s13203-013-0030-1
Goodman BA, Russell JD, Fraser AR, Woodhams FWD (1976) A Mössbauer and IR spectroscopic study of the structure of nontronite. Clays Clay Miner 24:53–59
Gueu S, Finqueneisel G, Zimny T, Bartier D, Yao BK (2019) Physicochemical characterization of three natural clays used as adsorbent for the humic acid removal from aqueous solution. Adsorpt Sci Technol 37:77–94. https://doi.org/10.1180/clm.2020.26
Gueu S, Yao B, Adouby K, Ado G (2006) Heavy metals removal in aqueous solution by activated carbons prepared from coconut shell and seed shell of the palm tree. JApSc 6:2789–2793. https://doi.org/10.3923/jas.2006.2789.2793
He L-J, Zheng S-Q, Dai Y-L (2017) Povećanje iskorištenja benzina katalizatorom za krekiranje u fluidiziranom sloju (FCC). Kemija industriji 66:9–15
Houdry EJ (1953). Process for catalytically cracking hydrocarbons
Hussain AI, Aitani AM, Kub\uuČejkaAl-Khattaf MJS (2016) Catalytic cracking of Arabian light VGO over novel zeolites as FCC catalyst additives for maximizing propylene yield. Fuel 167:226–239
Ibarra Á, Hita I, Azkoiti MJ, Arandes JM, Bilbao J (2019) Catalytic cracking of raw bio-oil under FCC unit conditions over different zeolite-based catalysts. J Ind Eng Chem 78:372–382. https://doi.org/10.1016/j.jiec.2019.05.032
Karimi N (2007) Etude par diffraction des rayons X in situ des mécanismes d’oxydation de l’acier AISI 304 entre 800 °C et 1000 °C. Influence des dépôts sol-gel de lanthane et de cérium. Apport de la spectroscopie infrarouge à l’identification des oxydes mixtes (PhD Thesis)
Leprince P (1998) Le raffinage du pétrole: procédés de transformation. Technip, Paris, p 550
Liu Z, Zhang Z, Yang C, Gao X (2015) Domestic technology developments on high-efficiency heavy oil conversion FCC catalysts’ industrialization. Appl Petrochem Res 5:269–275. https://doi.org/10.1007/s13203-015-0133-y
Mamudu A, Emetere M, Okocha D, Taiwo S, Ishola F, Elehinafe F, Okoro E (2020) Parametric investigation of indigenous Nigeria mineral clay (Kaolin and Bentonite) as a filler in the Fluid Catalytic Cracking Unit (FCCU) of a petroleum refinery. Alexandria Eng J 59:5207–5217. https://doi.org/10.1016/j.aej.2020.09.050
Murray HH (2006) Applied clay mineralogy: occurrences, processing and applications of kaolins, bentonites, palygorskitesepiolite, and common clays. Elsevier
Nguyen TP, Jonnard P, Vergand F, Staub PF, Thirion J, LapkowskiTran MVH (1995) Characterization of the poly (para-phenylene vinylene)-chromium interface by attenuated total reflection infrared and X-ray emission spectroscopies. Synth Met 75:175–179
Olaremu AG, Adedoyin WR, Ore OT, Adeola AO (2021) Sustainable development and enhancement of cracking processes using metallic composites. Appl Petrochem Res. https://doi.org/10.1007/s13203-021-00263-1
Otmani S (2006) Valorisation des charges lourdes compoundées par le craquage catalytique (PhD Thesis)
Pouwels C, Bruno K (2013) FCC catalyst design evolves to maximize propylene. Hydrocarbon Process
Qureshi MS, Nisar S, Shah R, Salman H (2020) Studies of liquid fuel formation from plastic waste by catalytic cracking over modified natural clay and nickel nanoparticles 10. Pakistan J Sci Ind Res 63(2):79
Ribeiro AM, Machado Júnior HF, Costa DA (2013) Kaolin and commercial fcc catalysts in the cracking of loads of polypropylene under refinary conditions. Braz J Chem Eng 30:825–834
Saravani H, Khajehali M (2016) Synthesis and characterization of lanthanum oxide and lanthanumoxid carbonate nanoparticles from thermalizes of [La(acacen)(NO3)(H2O) complex. Orient J Chem 32:491–498. https://doi.org/10.13005/ojc/320156
Sun F (2010) Caractérisation de revêtements de silicate de lanthane de structure apatite dopé au magnésium réalisés par projection plasma en vue d’application comme électrolyte de pile à combustible de type IT-SOFC (PhD Thesis)
Zhang Z, Liu Z, Feng R, Liu P, Yan Z (2014) The development of FCC catalysts for producing FCC gasoline with high octane numbers. Appl Petrochem Res 4:379–383
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdoulaye Dan Makaou, O., Gueu, S., Gourouza, M. et al. Development of semi-synthetic catalyst based on clay and their use in catalytic cracking of petroleum residue. Appl Petrochem Res 11, 147–154 (2021). https://doi.org/10.1007/s13203-021-00268-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-021-00268-w