Abstract
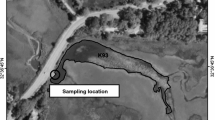
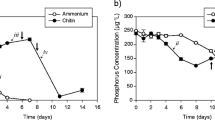
At a paper manufacturing mill (PML) that had been previously part of an integrated pulp (bisulphite) and paper mills industry, cyanobacterial blooms were observed in the mill’s aerated stabilization basin (ASB) ponds at about 3 years after pulping had been discontinued. This study aimed to determine the factors that led to bloom occurrences and potential control strategies applicable to the paper manufacturing mill’s, aerated stabilization basin system that discharges wastewaters into a coastal lake. Following discontinuation of pulping, the colour of the wastewaters reduced to low levels (~ 10 Hazen units or less), while the levels of nutrient remained potentially supportive of cyanobacteria growth with total phosphorus at ~ 0.1 mg/L and total nitrogen at > 2.5 mg/L. Incidences of blooms in the ABS were associated with preceding average monthly rainfalls and wind speeds being less (~ 44% and ~ 7%, respectively) and average direct sunlight hours being greater (~ 9%) than the long-term average values. Zinc was investigated for control of cyanobacteria as this metal is less toxic to aquatic organisms and microflora than copper algaecides. In laboratory culture trials, zinc was found to inhibit growth of M. aeruginosa (strain MIC338) and Pseudanabaena sp. when dosed at ~ 2.5 mg/L. The inhibition of cyanobacteria by zinc was found to vary between ASB pond samples which had different in situ chlorophyll a levels. This study found that the PML wastewaters investigated can be supportive of cyanobacterial growth to bloom levels after discontinuation of pulping processes and that zinc has potential as a cyanobacteria control agent.
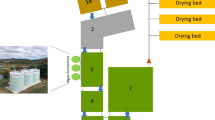
Graphic abstract





Similar content being viewed by others
Availability of data and material
Primary data of research reported in this manuscript will be made available upon request. Some key primary data are provided in Supplementary information. The research reported in this manuscript is part of a postgraduate study and primary data will be made available through University of South Australia public access to the finalized thesis.
Code availability
Not applicable.
Abbreviations
- PML:
-
Paper mill
- HU:
-
Hazen units
- TAL sensor:
-
EXO total algae PC smart sensor
- AWQC:
-
Australian Water Quality Centre
- LPP:
-
Large-scale pilot plant
- SPP:
-
Small-scale pilot plant
- ANZECC:
-
Australian and New Zealand Environment and Conservation Council
- IPc:
-
Initial phycocyanin
- FS ASB:
-
Full-scale ASB
- ASB:
-
Aerated stabilization basin
References
Anderson B et al (2018) Extracts from benthic anatoxin-producing phormidium are toxic to 3 macroinvertebrate taxa at environmentally relevant concentrations. Environ Toxicol Chem 37:2851–2859
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, vol 1. Canberra, pp 1–103
Aslam Z, van Leeuwen J, Neil Crossing N (2015) Model development for prediction of alum dosing for treatment of domestic wastewater for recycling purposes. Paper presented at the 21st International Congress on Modelling and Simulation (MODSIM2015), Gold Coast, Queensland, Australia, 29 November–4 December
Bartram J, Chorus I (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. CRC Press, Boca Raton
Bertone E, Chuang A, Burford MA, Hamilton DP (2019) In-situ fluorescence monitoring of cyanobacteria: laboratory-based quantification of species-specific measurement accuracy. Harmful Algae 87:1–14
Burch MD (2001) Cyanobacterial toxins—the Australian perspective on guidelines and management. In: Proceedings of 2001 AWWA WQTC, Nashville, sTenn
Burch MD, Chow CW, Hobson P (2001) Algicides for control of toxic cyanobacteria. In: Proceedings of the American water works association water quality technology conference, Nashville, TN, November 11–14 2001
Burger H, Awad J, Hewa GA, van Leeuwen J (2019) Simulation of the suppressive effect of zinc on cyanobacteria in paper mill wastewater. Paper presented at the MODSIM2019, 23rd International Congress on Modelling and Simulation. Modelling and Simulation Society of Australia and New Zealand, Canberra, Australia, December 2019
Carmichael WW, Boyer GL (2016) Health impacts from cyanobacteria harmful algae blooms: implications for the North American great lakes. Harmful Algae 54:194–212
Carvalho L et al (2013) Sustaining recreational quality of European lakes: minimizing the health risks from algal blooms through phosphorus control. J Appl Ecol 50:315–323
Casanova MT, Burch MD, Brock MA, Bond PM (1999) Does toxic Microcystis aeruginosa affect aquatic plant establishment? Environ Toxicol Int J 14:97–109
Chaffin JD, Bridgeman TB (2014) Organic and inorganic nitrogen utilization by nitrogen-stressed cyanobacteria during bloom conditions. J Appl Phycol 26:299–309. https://doi.org/10.1007/s10811-013-0118-0
Drikas M, Chow CW, House J, Burch MD (2001) Using coagulation, flocculation, and settling to remove toxic cyanobacteria. J Am Water Works Assoc 93:100–111
Dubey SK, Dubey J, Viswas A, Tiwari P (2011) Studies on Cyanobacterial biodiversity in paper mill and pharmaceutical industrial effluents . Biotechnol J Int 3:61–67
Dutta P, Bhakta S, Bastia A (2019) Growth response and biochemical composition of waste grown cyanobacteria to paper mill effluents Indian. Hydrobiology 18:156–163
Ebrahimpour M, Alipour H, Rakhshah S (2010) Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicol Ind Health 26:361–365
Falconer IR (2001) Toxic cyanobacterial bloom problems in Australian waters: risks and impacts on human health. Phycologia 40:228–233
Fu Z et al (2016) Copper and zinc, but not other priority toxic metals, pose risks to native aquatic species in a large urban lake in Eastern China. Environ Pollut 219:1069–1076. https://doi.org/10.1016/j.envpol.2016.09.007
L Garcı́a-Villada, M Rico, M Altamirano, L Sánchez-Martı́n, V López-Rodas, E Costas (2004) Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: characterisation and future implications in the use of copper sulphate as algaecide. Water Res 38:2207–2213
Gorham P, McLachlan J, Hammer U, Kim W (1964) Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) De Breb Verh. Internal Verein Limnol 19:796
Gündoğdu A (2008) Acute toxicity of zinc and copper for rainbow trout (Onchorhyncus mykiss). J. FisheriesSciencescom 2:711–720
Gupta RC (2015) Handbook of toxicology of chemical warfare agents. Academic Press, Cambridge
Hinners J, Hofmeister R, Hense I (2015) Modeling the role of pH on Baltic Sea cyanobacteria. Life 5:1204–1217. https://doi.org/10.3390/life5021204
Hodges CM, Wood SA, Puddick J, McBride CG, Hamilton DP (2018) Sensor manufacturer, temperature, and cyanobacteria morphology affect phycocyanin fluorescence measurements. Environ Sci Pollut Res 25:1079–1088
Hötzel G, Croome R (1999) A phytoplankton methods manual for Australian fresh-waters. Land and water resources research and development corporation, Canberra. ISBN 0642267715, Occasional paper series (Land and water resources research and development corporation (Australia)); No. 99/22
Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JM, Visser PM (2018) Cyanobacterial blooms. Nat Rev Microbiol 16:471
ISO HSM (1992) Water quality. measurement of biochemical parameters. Spectrometric determination of the chlorophyll-a concentration international organization for standardization (10260: 1992)
Jakubowska N, Szeląg-Wasielewska E (2015) Toxic picoplanktonic cyanobacteria. Mar Drugs 13:1497–1518
Ji X, Verspagen JM, Van de Waal DB, Rost B, Huisman J (2020) Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Sci Adv 6:eaax2926
Kirkwood AE, Nalewajko C, Fulthorpe RR (2001) The occurrence of cyanobacteria in pulp and paper waste-treatment systems. Can J Microbiol 47:761–766
Lewandowska AU, Śliwińska-Wilczewska S, Woźniczka D (2017) Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea. Mar Pollut Bull 125:30–38
Lewis R et al (2018) Study of the impacts of process changes of a pulp and paper mill on aerated stabilization basin (ASB) performance. Chemosphere 211:767–774
Li J, Hansson L-A, Persson KM (2018) Nutrient control to prevent the occurrence of cyanobacterial blooms in a eutrophic lake in southern Sweden, used for drinking water supply. Water 10:919
Li XF, Wang PF, Feng CL, Liu DQ, Chen JK, Wu FC (2019) Acute Toxicity and hazardous concentrations of zinc to native freshwater organisms under different pH values in China. Bull Environ Contam Toxicol 103:120–126. https://doi.org/10.1007/s00128-018-2441-2
Lu J, Zhu B, Struewing I, Xu N, Duan S (2019) Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep. 9:2480. https://doi.org/10.1038/s41598-019-38481-2
MacGregor BJ et al (2001) Microbiological, molecular biological and stable isotopic evidence for nitrogen fixation in the open waters of Lake Michigan environmental. Microbiology 3:205–219. https://doi.org/10.1046/j.1462-2920.2001.00180.x
Mahmood T, Paice M (2006) Aerated stabilization basin design and operating practices in the Canadian pulp and paper industry. J Environ Eng Sci 5:383–395
Okmen G, Bozanta E, Aysel U, Ceyhan N (2011) Zinc effect on chlorophyll a, total carbohydrate, total protein contents and biomass of cyanobacterial species. J Appl Biol Sci 5:67–73
Olano H, Martigani F, Somma A, Aubriot L (2019) Wastewater discharge with phytoplankton may favor cyanobacterial development in the main drinking water supply river in Uruguay. Environ Monit Assess 191:146
Olvera-Ramírez R, Centeno-Ramos C, Martínez-Jerónimo F (2010) Efectos tóxicos de Pseudanabaena tenuis (Cyanobacteria) en los cladóceros Daphnia magna y Ceriodaphnia dubia. Hidrobiológica 20:203–212
Paerl HW et al (2016) Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54:213–222
Paerl HW, Havens KE, Xu H, Zhu G, McCarthy MJ, Newell SE, Scott JT, Hall NS, Otten TG, Qin B (2020) Mitigating eutrophication and toxic cyanobacterial blooms in large lakes: the evolution of a dual nutrient (N and P) reduction paradigm. Hydrobiologia 847:4359–4375
Perez JL, Chu TJ (2020) Effect of zinc on Microcystis aeruginosa UTEX LB 2385 and its toxin production. Toxins 12:92
Pineda-Mendoza RM, Olvera-Ramirez R, Martínez-Jerónimo F (2012) Microcystins produced by filamentous cyanobacteria in urban lakes. A case study in Mexico City. Hidrobiológica 22:290–298
Polyak Y, Zaytseva T, Medvedeva N (2013) Response of toxic cyanobacterium Microcystis aeruginosa to environmental pollution water. Air Soil Pollut 224:1494
Romo S, Soria J, Fernandez F, Ouahid Y, Barón-Sola Á (2013) Water residence time and the dynamics of toxic cyanobacteria. Freshw Biol 58:513–522
Singh VP, Saxena PN (1969) Preliminary studies on algal succession in raw and stabilized sewage. Hydrobiologia 34:503–512. https://doi.org/10.1007/BF00045406
Sklenar K, Westrick J, Szlag D (2016) Managing sals. American Water Works Association and Water Research Foundation, Denver
Tsai K-P (2016) Management of target algae by using copper-based algaecides: effects of algal cell density and sensitivity to copper water. Air Soil Pollut 227:1–11
US EPA USEPAUE (2020) Control measures for cyanobacterial HABs in surface water. US EPA. https://www.epa.gov/cyanohabs/control-measures-cyanobacterial-habs-surface-water. Accessed 22 Dec 2019
Watson SB, Ridal J, Zaitlin B, Lo A (2003) Odours from pulp mill effluent treatment ponds: the origin of significant levels of geosmin and 2-methylisoborneol (MIB). Chemosphere 51:765–773. https://doi.org/10.1016/S0045-6535(03)00030-4
Willis BE, Pearce M, Bishop WM (2018) Evaluation of copper dissipation, exposure factor, and algaecidal efficacy in an irrigation canal following pulse ‘slug’application of a chelated copper algaecide. Appl Water Sci 8:194
Wiltsie D, Schnetzer A, Green J, Vander Borgh M, Fensin E (2018) Algal blooms and cyanotoxins in Jordan Lake. North Carolina Toxins 10:92
Wu H, Lin L, Shen G, Li M (2017) Heavy-metal pollution alters dissolved organic matter released by bloom-forming Microcystis aeruginosa. RSC Adv 7:18421–18427
Xu K, Juneau P (2016) Different physiological and photosynthetic responses of three cyanobacterial strains to light and zinc. Aquat Toxicol 170:251–258
Zeng J, Wang W-X (2011) Temperature and irradiance influences on cadmium and zinc uptake and toxicity in a freshwater cyanobacterium, Microcystis aeruginosa. J Hazard Mater 190:922–929
Zeng J, Yang L, Wang W-X (2009) Cadmium and zinc uptake and toxicity in two strains of Microcystis aeruginosa predicted by metal free ion activity and intracellular concentration. Aquat Toxicol 91:212–220
Zhou S, Shao Y, Gao N, Deng Y, Qiao J, Ou H, Deng J (2013) Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Sci Total Environ 463:111–119
Acknowledgements
The authors acknowledge the financial support granted by Kimberly-Clark Australia Pty Ltd. and by the University of South Australia. The authors thank Mr. Malcolm Abbott for sample collections, wastewater quality analyses and support and Mr. Graham Burch for project direction, support and advice. We thank Professor Christopher Chow for his valuable advice on culturing of cyanobacteria. The support of Mr. Max Zimmerman and Mr. Cédric Dajnak in facilitating laboratory and fieldwork is gratefully acknowledged. We also thank the two reviewers for constructive comments that significantly improved the quality of the manuscript.
Funding
The research was supported by Kimberly-Clark Australia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study reported in the manuscript. Mr. Hugh Burger is the principal author and researcher of the work reported, which is of his Ph.D. dissertation; he wrote first and subsequent manuscript drafts following co-author reviews, data acquisition and analysis, modelling of data, methodology, concept and design. Dr. Sandy Dickson provided resources—cultures of MIC338, contributed to methodology, interpretation of findings, manuscript review and editing. Dr. John Awad provided supervision, guidance on data interpretation, modelling of data, contributed to writing, review and editing. Mr. Josef Marzouk contributed to resources, experimental work, methodology and review. Prof. John van Leeuwen, corresponding author, provided supervision, guidance on data interpretation, modelling concepts, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Editorial responsibility: Parveen Fatemeh Rupani.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burger, H., Dickson, S., Awad, J. et al. Investigation of cyanobacteria blooms in paper mill wastewaters and assessment of zinc as a control agent. Int. J. Environ. Sci. Technol. 19, 1105–1120 (2022). https://doi.org/10.1007/s13762-021-03198-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03198-1