Abstract
Metallurgical grade alumina is produced worldwide through the well-known Bayer process, which unavoidably generates bauxite residue (BR, also known as red mud) in almost equal amounts to alumina. This study aims the valorization of BR through a smelting-reduction process to obtain calcium aluminate slags that can be a proper feed for alumina recovery via the Pedersen process. It investigates the thermodynamics and characteristics of the slags and pig iron produced from mixtures of BR, a bauxite beneficiation byproduct, and lime. In this context, the evolution of the different phases in the slags is studied with advanced analytical techniques and thermodynamic calculations. According to the results, a CaO/Al2O3 mass ratio within 1.3 to 1.4 in the slags can yield more Al2O3-containing leachable phases, such as CaO·Al2O3 and 12CaO·7Al2O3. The cooling dictates the amount and the characteristics of these phases, and the slower cooling rate yields improved slag characteristics. The distribution of the elements between the slag and metal phases shows that iron is separated, and the majority of the P, Cr, Ni, and V are distributed in the produced pig iron, while S, Ti, and Si are mostly concentrated in the slags.
Similar content being viewed by others
Introduction
Alumina is the feedstock to produce aluminum and is mainly extracted from Bauxite ore.[1] Bauxites are composed mostly of alumina hydrate minerals and, in a lesser amount, hydrated aluminum silicate, iron, and titanium minerals.[2] The common industrial way to produce alumina is the well-known Bayer process,[3] in which large amounts of a residue, called bauxite residue (BR), are generated. As an example, 1 ton of alumina production can generate from 0.8 to 1.5 tons of BR (depending largely on the quality of the bauxite ore used).[3] BR holds all the insoluble/less-soluble compounds of bauxite ore in the Bayer leaching solution, containing Fe, Si, and Ti as the main elements, and some levels of Ca and Na (Na considers as solution loss) as well as valuable compounds as rare earth elements.[4,5] Extensive research has been done in recent years for the valorization of BR.[6,7,8,9,10,11,12] However, none of those attempts have been industrialized, commonly due to economic obstacles; hence, BR’s valorization is still challenging.
A different process for producing alumina was introduced at 1927 by Harald Pedersen, in Norway, and is considered as a method with no significant waste generation.[13,14] This process can be well adapted for the use of any bauxites; in particular, the ones with lower Al2O3/Fe2O3 ratio, which are not proper for the Bayer process.[13,14] The first step is the smelting reduction of bauxite ore with lime (CaO) as a flux and coke as a reducing agent.[15] The produced calcium-aluminate slag should contain phases that are suitable for further alkaline leaching to recover alumina. According to Azof et al., such phases, from higher to lower leachability, are 12CaO·7Al2O3 (C12A7), CaO·Al2O3 (CA), and 3CaO·Al2O3 (C3A).[16] According to Miller and Irgens, the formation of CaO·TiO2 (CT) from the input CaO and the TiO2 content of the raw materials will not retard the Al2O3 recovery.[13] The SiO2 content can be related with the input CaO to form 2CaO·SiO2 (C2S), which has three polymorphs: α, β, and γ-C2S. The transformation from β → γ-C2S will make the slag self-disintegrate as there is around 12 pct volume expansion.[17] The self-disintegration of the slag will be beneficial in terms of energy consumption for crushing prior to the leaching process. The formation of Al-containing nonleachable phases, particularly Gehlenite (2CaO·Al2O3·SiO2., C2AS), should be avoided since they will reduce the alumina recovery. The iron byproduct of the process can be used in the foundry and steel industry if the characteristics, such as the S content, are appropriate for further use.[14] The solid residue from the leaching step, called grey mud, can be used in cement and fertilizer production if it is classified as an inert material.[13,14,18]
Significant experimental and theoretical work has been done about the leachability of the calcium aluminate slags in the framework of the Norwegian Centre SFI-Metal production[19] and ENSUREAL EU project.[20,21,22] According to laboratory and pilot results, the slags can be used for alumina extraction if their major mineralogical Al2O3-containing phases are classified as leachable. In the current study, a BR and a calcite-rich bauxite beneficiation byproduct (BBBP) are used as raw materials for the Pedersen process. This study aims to valorize industrial wastes from the mining and alumina industry for iron and alumina recovery. The pyrometallurgical part of the Pedersen process and the optimization of the smelting reduction conditions are experimentally and theoretically studied. The research is supported by thermodynamic calculations and the application of significant material characterization techniques.
Materials and Methods
Raw Materials
BR powder and the calcite-rich BBBP were supplied by Mytilineos Metallurgy Business Unit S.A (former Aluminium of Greece). The BBBP is generated from the Greek (karstic) bauxite ore in order to remove carbonate minerals from the ore entering the Bayer process. Therefore, we emphasize that its availability depends on geographical parameters, and for the one used in this work, a considerable amount is produced every year that can be valorized by the BR as is studied in this work. The BBBP was received in big lumps and, thus, was crushed and then milled using a ring mill to reduce its particle size. The size reduction of the BBBP to lower than 500 μm (sieve size) was done to minimize the inhomogeneities along the ore particles. An additional reason was to achieve a better sintering, contact, and thus a bigger surface area of the raw materials during heating and, therefore, faster smelting and slag formation. In addition, lime was used as a flux with 95.5 pct purity (CaO wt pct) and was received in powder form. The particle size distribution of the dried powder materials was determined using a laser particle size analyzer, Horiba Partica LA-950. The D90 values of BR, BBBP, and lime were 1.25, 481, and 178.5 μm, respectively. Different mixtures were prepared from dried samples to achieve the desirable ratios between CaO to Al2O3, SiO2, and TiO2, based on mass balance calculations, as shown in Table I.
The chemical analysis of these materials was carried out by X-ray fluorescence (XRF). In Table II, the compositions of the raw materials in dry basis and their LoI are presented. Mineralogical analysis of the raw materials was done by X-ray diffraction (XRD) analysis using a Bruker D8 A25 DAVINCI™, with CuKα radiation, in the 2θ range of 10 to 75 deg diffraction wavelength and 0.02 deg step size.
Experimental Setup and Smelting-Reduction Procedure
The smelting reduction experiments were conducted in a graphite crucible in a 75-kVA open induction furnace. The input mixtures were heated to 1650 °C ± 20 °C, and the temperature and power were automatically controlled. The holding time at the target temperature was 90 minutes to ensure complete reduction of iron oxides in addition to proper slag and metal separation. The temperature was recorded with a thermocouple type C placed in the charged material. After the smelting-reduction trials, the metal settled in the bottom of the crucible due to the significant density difference with the slag, as schematically shown in Figure 1. However, study of the cross-sectioned crucibles after the trials indicated some tiny metal droplets on the side walls of the crucible.
As seen in Table I, the mixtures S-1 and S-2 have the same mixing analogies of BR and BBBP, but S-2 has higher CaO/Al2O3 to study the effect of lime addition. The higher CaO/Al2O3 ratio (mixtures S-2 and S-3) was designed to form C2S and CT along with C12A7 in the produced slags, based on mass balance calculations and our previous works.[23,24] The mixtures S-3, S-3-S, and S-3-R have the same mixing analogies and added lime, while the studied parameter is the effect of the applied cooling rate. The used mass of the BR and BBBP was totally 1 kg.
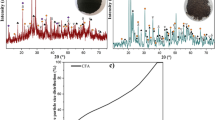
The samples S-1 through S-3 were slowly cooled from 1650 °C to 1350 °C ± 20 °C with a rate of 10 °C/min based on the programmed temperature profile. After 30 minutes at 1350 °C, the crucible was left in the furnace to be cooled to room temperature. The different cooling rates measured in samples S-3, S-3-S, and S-3-R are shown in Figures 2(a) and (b). For S-3-S, the furnace was shut down after the dwelling time at 1650 °C and the sample was left to be cooled inside the furnace. For S-3-R, the crucible was taken out from the top open hot furnace (after the dwelling time at 1650 °C) and was cooled outside of the furnace in air. The cooling rates that are reported in Table I are based on the average measured cooling rates from the thermocouple readings that were embedded in the sample. In addition, two thermocouples were used in experiment S-3-R in the bottom of the crucible and 5 cm above (in the molten bath) to examine any temperature gradient. As shown in Figure 2(b), the temperature is slightly higher at the bottom but the temperature difference is considered negligible. This is due to almost uniform induction into the crucible wall and then heat transport into the materials.
After solidification and cooling to room temperature, the crucibles were cut in half to separate the slag and metal. Half of the slag samples were milled to a powder and analyzed by XRF and XRD, while large slag particles from the second half were mounted in epoxy and then ground and polished using SiC emery papers. They were carbon coated for studying the quantitative elemental distribution in the slag phases using a JXA-8500F™ electron probe microanalyzer (EPMA), supported by wavelength dispersive spectroscopy (WDS). The metal samples were also analyzed with XRF and EPMA techniques.
Theoretical Calculations
Theoretical calculations were conducted using FactSage 7.3 thermochemical software to support the experimental design and evaluate the obtained results.[25] The compositions of the molten slags and metals were predicted through the equilibrium module based on the raw material compositions and using the commercial FToxid, FSStel, and FactPS databases. Scheil–Gulliver cooling calculations were conducted subsequently on the predicted liquid compositions for the phase distribution in the slags. The main assumptions of the Scheil–Gulliver cooling model have been presented extensively in other articles[25,26] and they are not repeated here. A dedicated EnsureAl database was also used to minimize the lack of data in the commercial databases as it covers the metastable C12A7 phase. It is worth noting that the considered thermodynamic properties of the C12A7 have been based on the phase equilibrium data that are available in the literature.[27]
Results
Characteristics of Raw Materials
The XRD analysis of the raw materials (Figure 3) shows that iron presents as hematite (Fe2O3) and, in a lower amount, as goethite (FeOOH). Titanium appears as anatase (TiO2) and calcium as calcite (CaCO3). Aluminum is found in hydroxides as gibbsite AlO(OH)3, diaspore α-AlO(OH), and boehmite β-AlO(OH), in complex alumino-sodium-silicate phases as Na1.15Al1.15Si0.85O4 and cancrinite (Na6Ca2(AlSiO4)6(CO3)2(H2O)2), and as katoite (Ca3Al2(SiO4)(OH)8) in the BR.
Table II shows the XRF analysis of the raw materials used in the present study. BR contains a significant amount of Fe2O3, 43.59 wt pct, while the alumina content is 22.78 wt pct, indicating levels of alumina losses that occur in the Bayer process.
Characteristics of Slags
Phase identification
The XRD results of the produced slags are illustrated in Figures 4 and 5. It was observed that there are significant differences compared to the mineralogical compositions of the raw materials (Figure 3) in the smelting reduction. Obviously, there are no Fe- and Na-containing phases in the produced slags. According to Figure 4, the main phases in slag S-1 are C2AS and CA. Weak peaks of C12A7 and CT were also detected.
C12A7 is the dominant phase for slag S-2, while larnite, β-C2S, CT, CA, and C2AS were also detected. Slag S-3 contains C12A7, β-C2S, CT, CA, and weaker peaks of C2AS in comparison with slags S-1 and S-2. The carbon detected in all the mentioned slags was due to the contamination from the graphite crucibles and thermowell.
The XRD results of the S-3 with different cooling paths are given in Figure 5. The slag S-3-S contains C12A7, CT, C3A and C2AS. The slag S-3-R contains C2AS, CT, C3A, β-C2S and C12A7. The peaks of C2AS are obviously weaker in the S-3-S slag in comparison with the S-3-R, while C12A7 has a stronger peak in S-3-S than S-3-R.
The other minor phases, such as Ca3Ti2O7, anorthite CA2S, and CaTi2O4, which may form in the slags, could not be identified precisely due to their low intensity peaks in addition to the overlapping of several phases.
Chemical composition
According to the XRF analysis in Table III, the slags were composed mostly of CaO, Al2O3, SiO2, and TiO2 oxides. Slightly lower SiO2 content than expected was observed for S-3-S (in comparison with S-3 and S-3-R), which may be attributed to some inhomogeneities in the used raw materials or little fluctuation in temperature at elevated temperatures.
The XRF analysis reports the total iron content as Fe2O3; however, as will be discussed in Section 3, the Fe content of the slags is attributed to metallic entraps, based on the microstructural analysis. To further justify this, magnetic separation of iron by a normal magnet was applied in the S-3-R sample prior to its analysis. As seen in Table III, the Fe content was significantly reduced. Hence, the Fe2O3 content of the XRF has been transformed to metallic Fe in Table III.
Microstructural analysis
As the WDS results are more reliable than those using EDS for the determination of the chemical composition of the phases, we present the WDS results. In Figure 6 and Table IV, the BSE images and WDS analysis of the phases in slags S-1 through S-3 and S-3-R in different locations are presented. We emphasize that we observed even distribution of SiO2 in the main phases (overall distribution), mostly for samples S-2, S-3-S, and S-3-R. However, the compositions of the same phases in different samples were observed to be different regarding the content of the impurities (SiO2 and TiO2) in the calcium aluminate phases, as seen in the WDS analysis results in Table IV.
In slag S-1, three phases were observed and marked as A, B, and C. The continuous phase A that appears as light gray is attributed to the C2AS phase. The dark gray phase B is rich in CaO and Al2O3, and its ratio corresponds to CA2, not CA as detected with the XRD analysis. The bright-phase C has a high concentration of CaO and TiO2, which could be attributed to the CT phase, but it contains 10.8 wt pct Al2O3. However, a phase like this was not observed in the XRD analysis. According to Dunyushkina et al., Al ions could incorporate in the CT structure even if this phase appears as CT in the XRD spectra.[28] In slag S-2, phase A (dark gray) has an acicular shape and the C/A mass ratio is close to the theoretical mass ratio of C12A7; however, it contains some Si and Ti impurities. It is worth noting that based on the recent work by Azof, this phase may be C12A7 with Si ions in the locations of some Al ions and is so called “Si-mayenite.”[29] The solidified structure B seems to be a mixture of small C2S, CT, and possible CA phases distributed nonuniformly. The fine structure phase C may be the C12A7 phase containing Si impurities.
In slag S-3, the bright phase A (Figure 6 and Table IV) was rich in TiO2 and CaO, as in S-1, it was high in Al2O3; in the XRD analysis, no Ca-Al-Ti-O phase was observed. The light gray phase B is composed of CaO and Al2O3, with the C/A mass ratio similar to the C12A7. Sulfur has a higher concentration in comparison with the rest of the phases, and based on the literature, the C12A7 phase dissolves S in its structure.[30] Thus, we may claim that this phase is the C12A7 phase, as identified in the XRD analysis. The solidified structure C could be a mixture of C12A7, C2S, and CT, which supports the XRD result. The measured analysis of phase D was difficult to evaluate due to that phase’s size and morphology. However, based on WDS and XRD analysis, it could be a mixture of C2AS, C12A7, and C2S phases, but the identification is not precise in this case. It is observed that metallic iron particles were entrapped in the slag structure, as seen in Figure 6 for slag S-3.
The detected phases in the S-3-S and S-3-R slags were found to be impure, as SiO2 and TiO2 were detected in all the analyzed points. This is possibly due to the increased cooling rate, in comparison with the S-3 slag. In the S-3-R sample, it is found that the rapid cooling rate is accompanied with the formation of porosity (Figure 6). Phase A is likely a mixture of C12A7, CT, and C2AS, but it is evidenced that Si is evenly dispersed. Phase B could be the C2AS phase detected in the XRD analysis but seems not to be stoichiometrically the same. For clarity, the WDS results of slag S-3-S are not included.
Characteristics of Pig Iron
Chemical composition
The chemical analysis of the pig iron samples (Table V) shows that the Fe content of all the metals is ~92 wt pct. The Si content is low and varies between 0.22 and 0.02 wt pct, while the titanium concentration varies from 1.4 to 0.38 wt pct. Cu did not appear in the chemical composition of the raw materials (Table II), possibly due to its low concentration, while we see a small amount of it in the metal phase.
Microstructure
Typical examples of the micrographs of the metallic phase are given in Figure 7. The microstructural analysis of the produced pig irons indicates that they have a relatively similar microstructure, where the graphite flakes are distributed in the matrix, as seen in Figure 7.
At the surface of the metallic phase and mostly close to the graphite flakes, complex carbides were formed that primarily consisted of titanium and vanadium as indicated with the mapping. However, Si and Cr elements were found to be evenly distributed in the Fe matrix. In the metallic phase, M-3-R was observed with less carbides formed in comparison with M-3. This is possibly due to less Ti in the M-3-R (Table V) in addition to the applied cooling, as will be discussed subsequently.
Theoretical and Modeling Calculations
Equilibrium study and distribution of elements
The predicted equilibrium compositions of metal and slag phases in the molten state are listed in Table VI. The calculations were carried out at the experimental temperature of 1650 °C, and the metal phase was assumed to be carbon saturated. As seen in Table VI, a relatively good agreement was observed between the calculated and experimental (Table III) contents of Al2O3 and CaO. Besides, a higher but not significant difference was observed for the SiO2 and TiO2. Moreover, FactSage predicts that parts of TiO2 and Cr2O3 will be reduced to Ti2O3 and CrO, respectively.
Scheil–Gulliver cooling calculations
The Scheil–Gulliver cooling calculations are shown in Figure 8. The calculations were done based on the liquid slag compositions previously calculated (Table VI) concentrated in the main components (above 2 pct). The main phases that appear for S-1 in descending order are CA, C12A7, α-C2S, CaTi (ss) that is a Ca3Ti2O7 (s)-Ca3Ti2O6 (s) solid solution, PERO that is a perovskite Ca2Ti2O6 (s)-Ca2Ti2O5 (s) solid solution, and γ-C2S.
For slag S-2, the predicted phases are C12A7, α-C2S, CaTi (ss), C3A, and Ca3Ti2O7, and for slag S-3, they are C12A7, α-C2S, CA, CaTi (ss), C3A, and Ca3Ti2O7.
Discussion
Reduction Reactions and Distribution of Elements
In the present study, the applied temperature was 1650 °C to ensure higher reaction rates and proper slag-metal separation. The carbon from the graphite crucible ensures a high carbon activity in the system and presents as CO (g) gas and dissolved carbon in the liquid iron, C. In the applied temperature, it is expected that V, Cr, Ni, and Cu will be reduced to the metallic phase, while sodium oxide is reduced and Na will evaporate.[31] This has been confirmed through the chemical analysis of the slags and metals (Tables III and V). When solid carbon reacts with iron oxides, higher temperatures are required to reduce them to metallic iron in comparison with the reduction by CO (g) since they are less exothermic. The reduction of Fe2O3 to metallic iron occurs through steps Fe3O4 → FeO → Fe, and most likely the reduction to metallic iron occurs mainly through the FeO reduction of the slags[16,32] by both solid and dissolved carbon in liquid iron.
Partial Si reduction was observed from the measured compositions of the products (Table V and Figure 7). The mechanism for the Si transfer in the pig iron phase has been explained in relevant articles[32,33,−34] and is believed to occur through the reaction of SiO2 with C. Nevertheless, a small amount of Si is transferred into the metallic phase, as the SiO2 content of the charge mixtures is low; therefore, low activity of SiO2 in the slag is expected. A higher extent of SiO2 reduction in bauxite smelting has been observed in the case of the smelting reduction of high SiO2-containing bauxites (SiO2 > 20 pct).[16]
The chemical compositions of pig irons (Table V) revealed that some Ti has been transferred into the metallic phase. It was seen from the micrographs that Ti was not dissolved in the metallic phase but was precipitated as complex (Ti,V)xCy carbides, and based on earlier studies, their precipitation is likely to occur upon cooling and solidification.[15,16] In agreement with Morizane et al., the slower the cooling, the bigger in shape are the carbide precipitates, as seen in Figure 7.[35] Nevertheless, the effect of the anticipated characteristics should be investigated more in terms of the further use of the produced pig irons. The pig iron chemical compositions (Table V) revealed low content of S, and this is important for the subsequent processing of the pig iron.
An important aspect in the assessment of the slag/metal reaction extents is the equilibrium distribution coefficient, Li, of the different species, which is linked to the thermodynamic properties in the metal and slag phases. The distribution coefficient values were calculated based on Eq. [1], and the results (in logarithmic scale) are presented in Figure 9 using the measured compositions by XRF and calculated (FactSage) compositions[36,37] based on the charge materials characteristics.
where (wt pct i) is the content of element i in the slag and [wt pct i] is the content of element i in the metal. Species with negative log10Li are distributed mostly in the metal phase, while the species with positive log10Li are concentrated in the slag phase. Based on Figure 9, a similar trend was observed in the distribution behavior of the elements between the experimental results (S-1 through S-3), while the LS reveals a proper desulfurization to the slag, as also seen in Table III.
The Li coefficients determined for the experimental and calculated results show some differences. As mentioned, FactSage predicts that part of the TiO2 and Cr2O3 will be reduced to Ti2O3 and CrO, respectively (Table VI), while it overestimates the metallization of Si, Ti, and Cr, as it assumes thermodynamic equilibrium. It is worth noting that the oxidation states of Ti4+, Ti3+, Cr3+, and Cr2+ have been taken into consideration for the Li calculation. The oxidation states of Ti and Cr are strongly dependent on the oxygen potential that may be changed during solidification and deviate between the experimental conditions and equilibrium calculations. In addition, the distribution of Si and Ti (Table VI) will be defined based on both thermodynamic and kinetic parameters, but the model excludes the kinetic effects. In addition, the variations between the model assumptions and the experimental conditions (i.e., lower partial pressure of O2 in the presence of carbon, open furnace conditions, and kinetic effects), and uncertainties about the Ti content in the applied databases, make the observed differences reasonable. Nevertheless, further experimental and modeling assessment is necessary for further discussion of anticipated discrepancies.
Slag Characteristics
The results obtained for slags S-1 and S-2 clearly indicate that increasing the lime addition enhances the formation of the preferable phases, as also seen from the theoretical calculations in Figure 8. The formation of CT and C2S should be taken into consideration in terms of the slag chemistry and proper CaO additions. Slag S-3 was found to contain similar phases as slag S-2, and their C/A mass ratios are quite similar.
For slags S-3, S-3-R, and S-3-S, it was observed that the slower cooling rate, that is, ~10 °C/min (Table I), yields more C12A7 in the slag as the C12A7 phase is not dominant when the cooling rate becomes higher (~52 °C/min, Table I). Besides, the increased cooling rate is accompanied with the formation of less pure leachable phases with higher Si and Ti impurities, as illustrated by the WDS analysis in Table IV. The effect that this may have on the recovery of alumina can be the dissolution of Si in the leaching step that affects both further desilication of the sodium aluminate solution, which causes some Al loss, and the purity of the alumina product.[38] It is worth noting that based on the EPMA results (Figure 6) in all slags, the CT phase seems to incorporate Al in its structure, as observed in Reference 28, while in another study regarding the CaO-Al2O3-TiO2 system, the formation of Ca3Ti8Al12O37 is evidenced.[39] However, the formation of complex Ca-Ti-Al-O phase should be investigated more in addition to the effects it may have in Al2O3 leaching. Nevertheless, this case most likely occurs for high Ti concentrations in the raw materials and the obtained slag and is beyond the scope of the present study as we did not detect Ca3Ti8Al12O33 by the EPMA and XRD analysis.
Stabilization of C12A7
Both the experimental results and the theoretical calculations indicate that C12A7 is a stable phase at low temperatures. According to the literature, in both inert and dry atmosphere conditions, C12A7 will not form since it needs excess O2 to be stabilized; thus, an O2 containing atmosphere or small amount of moisture in an inert atmosphere can enhance the formation of C12A7.[40,41,42] The experiments in the current study were conducted in top open crucibles and in exposure to air with normal humidity; hence, the C12A7 phase is expected to appear in the slag samples. Nityanand and Fine studied the effect of TiO2 on the liquidus temperatures of CaO-Al2O3 as a function of the oxygen potential and concluded that TiO2 can have a similar effect as the water vapor in the C12A7 formation.[43] Azof has recently shown that the existence of a small amount of SiO2 in the CaO-Al2O3 slags has a similar effect, and they called a C12A7 phase that contains Si “Si-mayenite.”[29] Kim et al. observed the dissolution of sulfur in the C12A7 structure, as we also observed in the current study.[30] Based on the EPMA analysis of S-3 (Figure 6 and Table IV), we can see that the C12A7 phase has S in its structure. The free oxygen in the structure of C12A7 can be substituted by other anions, such as S, F–, Cl–, and OH–,[30,44,45,46] which can be characterized as stabilizing agents. Hence, we may claim that under the applied conditions, all the critical parameters for C12A7 stabilization (the excess O2, existence of moisture, TiO2, and S) have enhanced its formation. Nevertheless, regarding the complexity of the system, we cannot conclude which of the aforementioned parameters plays the most important role in C12A7 stabilization.
Effect of slag chemistry and cooling rate on C12A7 formation
Based on the anticipated experimental and theoretical results, we may claim that Ca diffusion plays an important role in the formation of several CxAy phases, in agreement with the literature.[47,48] According to the microstructure analysis in Figure 6 and Table IV, it seems that in slow cooling (slag S-3), the mayenite phase appears as a pure phase. Thus, in connection to the slag chemistry, it is possible for C12A7 to precipitate directly from the melt. On the other hand, based on the literature, it is possible that C3A reacts with Al2O3 to form C12A7 as C3A is less reactive and stable.[16,49] When we employed high cooling rates, C2AS and C3A were favored instead of C12A7. Based on the EPMA results, the impurities in the mayenite phase were increased (Si and Ti impurities in C12A7, slag S-3-S, and slag S-3-R) while increasing the cooling rate and the appearance of C2AS was evidenced. Consequently, we may claim that when the cooling rate becomes high, there is not enough time for complete diffusion of the species (in particular, Ca ions). Thus, it is possible that the remaining CA or Al2O3 will react with the coexisting C2S to form C2AS. This can be confirmed further from the XRD of slag S-3-S (Figure 5), where the C12A7, C2AS, C2S, and C3A coexist. The C2AS formation agrees with literature sources,[17] where during rapid cooling there is not enough time for diffusion depending on transformations and causing the C2AS. Hence, the C12A7 is formed only in a low concentration since its formation will not be kinetically favored, and C2AS, along with C3A, will be the dominant phase. The suggested mechanism here is schematically shown in Figure 10.
It is worth noting that the thermodynamic calculations did not predict the formation of C2AS since the present model excludes the kinetic effects, and we may point out that the formation of the different phases is highly dependent on the cooling rate. Our previous experimental work showed that the CaO/Al2O3 ratio must be in a range of 0.95 to 1.5 in the final slag.[23,24] Therefore, the operational parameters should be controlled regarding the target CaO/Al2O3 ratio and the cooling conditions. Based on the present study, applying slow cooling conditions and targeting a CaO/Al2O3 mass ratio of approximately 1.3 can provide a slag with proper characteristics for the subsequent alumina recovery. We intend to produce slags with higher amounts of Al2O3-containing leachable phases with the highest purities to attain the highest possible alumina recovery. The alumina recovery depends on both slag characteristics and later hydrometallurgical treatment. If Al is completely in the leachable phases, the Al recovery in the integrated process will be more than 90 pct.[50]
Conclusions
The carbothermic smelting reduction of a BR and a calcium-carbonate rich BBBP was done in order to separate the iron as pig iron and produce calcium-aluminate slags for further alumina recovery. The main conclusions drawn from the study are as follows.
-
1.
The iron oxide reduction and separation from the slags was properly done, yielding pig iron containing low concentrations of Si, S, P, Ti, V, and Cr. In the metal, Ti and V appear as complex carbide precipitates.
-
2.
It was found that the sodium oxide of the BR is completely reduced and removed from the system at elevated temperatures, yielding a Na-free slag.
-
3.
Both the experimental work and FactSage equilibrium calculations showed that the produced slags are richer in Si, Ti, and S than the metal, while the pig iron is richer in Cr. FactSage predicts much higher Si and Cr concentrations in the metal phase than do the experiments.
-
4.
The increase of the CaO/Al2O3 ratio enhanced the formation of the desirable leachable C12A7 and CA phases.
-
5.
It was observed that the cooling rate significantly affects the characteristics of the slags and the leachable C12A7 phase is obtained mainly under slower cooling rates.
-
6.
A possible mechanism for the formation of the C12A7 was suggested, in which the C12A7 is formed directly from the melt due to the C3A and Al2O3 reaction at slow cooling rates, while a high cooling rate interrupts the diffusion of the species, in particular Ca, and C2AS with C3A is formed instead.
References
R. Lumley: Fundamentals of Aluminium Metallurgy: Production, Processing and Applications, 1st ed., Woodhead Publishing Limited, Cambridge, United Kingdom, 2011, pp. 23–27.
J.T. Kloprogge, H.D. Ruan, and R.L. Frost: J. Mater. Sci., 2002, vol. 37 (6), pp. 1121–29.
E. Balomenos, D. Panias, and I. Paspaliaris: Miner. Process. Extr. Metall. Rev., 2011, vol. 32 (2), pp. 69–89.
J. Vind, A. Malfliet, C. Bonomi, P. Paiste, I.E. Sajó, B. Blanpain, and D. Panias: Min. Eng., 2018, vol. 123, pp. 35–48.
J. Vind, A. Malfliet, B. Blanpain, P.E. Tsakiridis, A.H. Tkaczyk, V. Vassiliadou, and D. Panias: Minerals, 2018, vol. 8 (2), p. 77.
P.E. Tsakiridis, S. Agatzini-Leonardou, and P. Oustadakis: J. Hazard. Mater., 2004, vol. 116 (1–2), pp. 103–10.
T. Hertel, B. Blanpain, and Y. Pontikes: J. Sustain. Metall., 2016, vol. 2 (4), pp. 394–404.
B. Xakalashe and B. Friedrich: Proc. 2nd Int. Bauxite Residue and Valorisation and Best Practices Conf., 2018, pp. 233–40.
F. Kaußen and B. Friedrich: Chem. Ing. Tech., 2015, vol. 87 (11), pp. 1535–42.
C.R. Borra, B. Blanpain, Y. Pontikes, K. Binnemans, and T. Van Gerven: J. Sustain. Metall., 2016, vol. 2 (1), pp. 28–37.
C. Cardenia, E. Balomenos, and D. Panias: J. Sustain. Metall., 2019, vol. 5 (1), pp. 9–19.
P.T. Yin, B.F. Buhle Xakalashe, D. Panias, and V. Vassiliadou: Proc. 35th Int. ICSOBA Conf., 2017, vol. 42, pp. 603–13.
J. Miller and A. Irgens: Light Metals, Springer, Cham, 1974, pp. 977–82.
J. Safarian and L. Kolbeinsen: Sustainable Industrial Processing Summit, 2016, pp. 75–82.
J. Safarian and L. Kolbeinsen: Sustainable Industrial Processing Summit, 2016, pp. 1–8.
F.I. Azof, L. Kolbeinsen, and J. Safarian: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2400–20.
W. Bo, S. Hui-lan, Z. Xue-zheng, and B. Shi-wen: Light Metals, Springer, Cham, 2011, pp. 241–44.
M. Vafeias, D. Marinos, D. Panias, J. Safarian, C. Van Der Eijk, I. Solhem, and P. Davris: Proc. 2nd Int. Bauxite Residue Valorisation and Best Practices Conf., 2018, pp. 111–17.
F.I. Azof, J. Safarian, and L. Kolbeinsen: Proc. 35th Int. ICSOBA Conf., 2017, vol. 42 (46), pp. 243–53.
F.I. Azof, Y. Yang, D. Panias, L. Kolbeinsen, and J. Safarian: Hydrometallurgy, 2019, vol. 185, pp. 273–90.
A. Lazou, C. Van der Eijk, E. Balomenos, and J. Safarian: Proc. EMC Conf., 2019, vol. 1 (17), pp. 17–34.
A. Lazou, C. Van der Eijk, E. Balomenos, L. Kolbeinsen, and J. Safarian: Proc. 3rd Int. Bauxite Residue Valorisation and Best Practices Conf., 2020, pp. 77–83.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, and S. Petersen: Comput. Coupl. Phase Diagr. Thermochem., 2016, vol. 54, pp. 35–53.
A.D. Pelton, G. Eriksson, and C.W. Bale: Metall. Mater. Trans. A, 2017, vol. 48A, pp. 3113–29.
B. Hallstedl: J. Am. Ceram. Soc., 1990, vol. 73 (1), pp. 15–23.
L.A. Dunyushkina, V.A. Gorbunov, A.A. Babkina, and N.O. Esina: Ionics, 2003, vol. 9 (1–2), pp. 67–70.
F.I. Azof: Pyrometallurgical and Hydrometallurgical Treatment of Calcium Aluminate-Containing Slags for Alumina Recovery, NTNU, Trondheim, Norway, 2020.
S.J. Kim, M. Kageyama, X. Gao, S. Ueda, and S.Y. Kitamura: ISIJ Int., 2019, vol. 59 (10), pp. 1752–55.
M. Hasegawa: Treatise on Process Metallurgy, 1st ed., Elsevier Ltd., Amsterdam, 2013, pp. 507–13.
J. Safarian, G. Tranell, L. Kolbeinsen, M. Tangstad, S. Gaal, and J. Kaczorowski: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 702–12.
N. Tsuchiya, M. Tokuda, and M. Ohtani: Metall. Mater. Trans. B, 1976, vol. 7 (3), pp. 315–20.
J. Safarian, L. Kolbeinsen, M. Tangstad, and G. Tranell: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 929–39.
Y. Morizane, B. Ozturk, and R.J. Fruehan: Metall. Mater. Trans. B, 1999, vol. 30B, pp. 29–43.
D. Shishin, T. Hidayat, U. Sultana, M. Shevchenko, and E. Jak: J. Sustain. Metall., 2020, vol. 6, pp. 68–77.
A.J. Andersson, Margareta T. Andersson, and P.G. Jönsson: Steel Res. Int., 2004, vol. 75 (5), pp. 294–301.
M.J. Mwase and J. Safarian: Proc. 38th Int. ICSOBA Conf., 2020, vol. 49, pp. 453–61.
K.T. Jacob and G. Rajitha: J. Phase Equilib. Diffus., 2012, vol. 33 (4), pp. 293–302.
J.A. Imlach, L.D. Glasser, and F.P. Glasser: Cem. Concr. Res., 1971, vol. 1 (1), pp. 57–61.
R.W. Nurse, J.H. Welch, and A.J. Majumdar: Trans. Br. Ceram. Soc., 1965, vol. 64 (9), pp. 409–18.
D.A. Jerebtsov and G.G. Mikhailov: Ceram. Int., 2001, vol. 27 (1), pp. 25–28.
N. Nityanand and H.A. Fine: Metall. Mater. Trans. B, 1983, vol. 14B, pp. 685–92.
J.P. Eufinger, A. Schmidt, M. Lerch, and J. Janek: Phys. Chem. Chem. Phys., 2015, vol. 17 (10), pp. 6844–57.
H. Boysen, M. Lerch, A. Stys, and A. Senyshyn: Acta Crystallogr., Sect. B: Struct. Sci., 2007, vol. 63 (5), pp. 675–82.
J. Jeevaratnam, F.P. Glasser, and L.D. Glasser: J. Am. Ceram. Soc., 1964, vol. 47 (2), pp. 105–06.
B.M. Mohamed and J.H. Sharp: Thermochim. Acta, 2002, vol. 388 (1–2), pp. 105–14.
M. Ruszak, S. Witkowski, P. Pietrzyk, A. Kotarba, and Z. Sojka: Funct. Mater. Lett., 2011, vol. 4 (02), pp. 183–86.
Y.Y. Zhang, W. Lü, Y.H. Qi, and Z.S. Zou: Int. J. Miner., Metall. Mater., 2016, vol. 23 (8), pp. 881–90.
F.I. Azof and J. Safarian: Hydrometallurgy, 2020, vol. 195.
Acknowledgments
This research was performed within the ENSUREAL project and received funding from the EU Horizon 2020 Program (H2020/2014-2020) under Grant Agreement No. 767533, which is gratefully acknowledged.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscripe submitted September 2, 2020; accepted January 14, 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lazou, A., Van Der Eijk, C., Tang, K. et al. The Utilization of Bauxite Residue with a Calcite-Rich Bauxite Ore in the Pedersen Process for Iron and Alumina Extraction. Metall Mater Trans B 52, 1255–1266 (2021). https://doi.org/10.1007/s11663-021-02086-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02086-w