Abstract
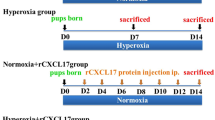
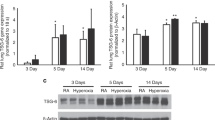
The proliferation of fetal alveolar type II cells (FATIICs) was impaired in bronchopulmonary dysplasia (BPD), which is modulated by hyperoxia and inflammatory response. Interleukin 24 (IL-24), a cytokine produced by certain cell types, plays an essential role in inflammation and host protection against infection. However, the ability of FATIICs to produce IL-24 remains unclear, and the role of IL-24 in BPD progression is yet to be determined. With reverse transcription quantitative polymerase chain reaction (RT-qPCR) and enzyme-linked immunosorbent assay, the authors evaluated whether FATIICs produce IL-24 in physiological conditions. The authors quantified IL-24 expression in the lungs of newborn rat pups exposed to hyperoxia (70% oxygen) and in FATIICs isolated on embryonic day 19 that were exposed to 95% oxygen or lipopolysaccharide (LPS). The role of IL-24 in FATIICs, cell proliferation, cell apoptosis, and cell cycle were further evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and flow cytometric analysis. Also, they assessed caspase-3 and SOCS3 mRNA in IL-24 siRNA-treated cells by using RT-qPCR. During culture, IL-24 mRNA and protein levels in FATIICs gradually decreased with FATIIC differentiation. IL-24 expression increased significantly in rat lungs exposed to hyperoxia and FATIICs exposed to oxygen or LPS. Recombinant IL-24 enhanced cell proliferation by decreasing the proportion of apoptotic cells and increasing the proportion of cells in the S phase. The IL-24 siRNA-treated cells expressed more caspase-3 mRNA. Furthermore, suppressor of cytokine signaling 3 (SOCS3) mRNA was significantly decreased in rats and FATIICs exposed to oxygen, whereas it dramatically increased in FATIICs exposed to LPS. The IL-24 siRNA-treated cells expressed more SOCS3 mRNA. These studies suggest IL-24 is a pulmonary target cytokine in BPD, and may possibly regulate SOCS3 in oxidative stress and inflammation of the lung.




Similar content being viewed by others
References
Morty, R. E. (2018). Recent advances in the pathogenesis of BPD. Seminars in Perinatology, 42(7), 404–412. https://doi.org/10.1053/j.semperi.2018.09.001.
Reiterer, F., Scheuchenegger, A., Resch, B., Maurer-Fellbaum, U., Avian, A., & Urlesberger, B. (2019). Bronchopulmonary dysplasia in very preterm infants: outcome up to preschool age, in a single center of Austria. Pediatrics International, 61(4), 381–387. https://doi.org/10.1111/ped.13815.
Cheong, J., & Doyle, L. W. (2018). An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Seminars in Perinatology, 42(7), 478–484. https://doi.org/10.1053/j.semperi.2018.09.013.
Endesfelder, S., Strauss, E., Scheuer, T., Schmitz, T., & Buhrer, C. (2019). Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respiratory Research, 20(1), 88 https://doi.org/10.1186/s12931-019-1063-5.
Velten, M., Heyob, K. M., Rogers, L. K., & Welty, S. E. (2010). Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. Journal of Applied Physiology (1985), 108(5), 1347–1356. https://doi.org/10.1152/japplphysiol.01392.2009.
Kandasamy, J., Rezonzew, G., Jilling, T., Ballinger, S. W., & Ambalavanan, N. (2019). Mitochondrial DNA variation modulates alveolar development in newborn mice exposed to hyperoxia. American Journal of Physiology—Lung Cellular and Molecular Physiology, https://doi.org/10.1152/ajplung.00220.2019.
Yee, M., Buczynski, B. W., & O’Reilly, M. A. (2014). Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells. American Journal of Respiratory Cell and Molecular Biology, 50(4), 757–766. https://doi.org/10.1165/rcmb.2013-0207OC.
Bao, T. P., Wu, R., Cheng, H. P., Cui, X. W., & Tian, Z. F. (2016). Differential expression of long non-coding RNAs in hyperoxia-induced bronchopulmonary dysplasia. Cell Biochemistry & Function, 34(5), 299–309. https://doi.org/10.1002/cbf.3190.
Menden, H. L., Xia, S., Mabry, S. M., Navarro, A., Nyp, M. F., & Sampath, V. (2016). Nicotinamide adenine dinucleotide phosphate oxidase 2 regulates LPS-induced inflammation and alveolar remodeling in the developing lung. American Journal of Respiratory Cell and Molecular Biology, 55(6), 767–778. https://doi.org/10.1165/rcmb.2016-0006OC.
Huusko, J. M., Karjalainen, M. K., Mahlman, M., Haataja, R., Kari, M. A., & Andersson, S., et al. (2014). A study of genes encoding cytokines (IL6, IL10, TNF), cytokine receptors (IL6R, IL6ST), and glucocorticoid receptor (NR3C1) and susceptibility to bronchopulmonary dysplasia. BMC Medical Genetics, 15(120). https://doi.org/10.1186/s12881-014-0120-7.
Mao, X., Qiu, J., Zhao, L., Xu, J., Yin, J., & Yang, Y., et al. (2018). Vitamin D and IL-10 deficiency in preterm neonates with bronchopulmonary dysplasia. Frontiers in Pediatrics, 6(246). https://doi.org/10.3389/fped.2018.00246.
Lee, H. S., & Lee, D. G. (2015). rIL-10 enhances IL-10 signalling proteins in foetal alveolar type II cells exposed to hyperoxia. Journal of Cellular and Molecular Medicine, 19(7), 1538–1547. https://doi.org/10.1111/jcmm.12596.
Madouri, F., Barada, O., Kervoaze, G., Trottein, F., Pichavant, M., & Gosset, P. (2018). Production of Interleukin-20 cytokines limits bacterial clearance and lung inflammation during infection by Streptococcus pneumoniae. EBioMedicine, 37 (417-427). https://doi.org/10.1016/j.ebiom.2018.10.031.
Garn, H., Schmidt, A., Grau, V., Stumpf, S., Kaufmann, A., & Becker, M., et al. (2002). IL-24 is expressed by rat and human macrophages. Immunobiology, 205(3), 321–334. https://doi.org/10.1078/0171-2985-00135.
Seong, R. K., Choi, Y. K., & Shin, O. S. (2016). MDA7/IL-24 is an anti-viral factor that inhibits influenza virus replication.Journal of Microbiology, 54(10), 695–700. https://doi.org/10.1007/s12275-016-6383-2.
Ross, B. X., Gao, N., Cui, X., Standiford, T. J., Xu, J., & Yu, F. X. (2017). IL-24 promotes Pseudomonas aeruginosa Keratitis in C57BL/6 mouse corneas. Journal of Immunology, 198(9), 3536–3547. https://doi.org/10.4049/jimmunol.1602087.
Andoh, A., Shioya, M., Nishida, A., Bamba, S., Tsujikawa, T., & Kim-Mitsuyama, S., et al. (2009). Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. Journal of Immunology, 183(1), 687–695. https://doi.org/10.4049/jimmunol.0804169.
Burmeister, A. R., Johnson, M. B., Yaemmongkol, J. J., & Marriott, I. (2019). Murine astrocytes produce IL-24 and are susceptible to the immunosuppressive effects of this cytokine. Journal of Neuroinflammation, 16(1), 55 https://doi.org/10.1186/s12974-019-1444-1.
Hasnain, S. Z., Borg, D. J., Harcourt, B. E., Tong, H., Sheng, Y. H., & Ng, C. P., et al. (2014). Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nature Medicine, 20(12), 1417–1426. https://doi.org/10.1038/nm.3705.
Liu, X. X., Yu, X. R., Jia, X. H., Wang, K. X., Yu, Z. Y., & Lv, C. J. (2013). Effect of hyperoxia on the viability and proliferation of the primary type II alveolar epithelial cells. Cell Biochemistry and Biophysics, 67(3), 1539–1546. https://doi.org/10.1007/s12013-013-9658-9.
Rao, X., Huang, X., Zhou, Z., & Lin, X. (2013). An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatistics, Bioinformatics and Biomathematics, 3(3), 71–85.
Stoll, B. J., Hansen, N. I., Bell, E. F., Walsh, M. C., Carlo, W. A., & Shankaran, S., et al. (2015). Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA, 314(10), 1039–1051. https://doi.org/10.1001/jama.2015.10244.
Madurga, A., Mizikova, I., Ruiz-Camp, J., Vadasz, I., Herold, S., & Mayer, K. et al. (2014). Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. American Journal of Physiology-Lung Cellular and Molecular Physiology, 306(7), L684–L697. https://doi.org/10.1152/ajplung.00361.2013.
Surate, S. D., Rodriguez-Castillo, J. A., Ahlbrecht, K., & Morty, R. E. (2017). Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. American Journal of Physiology-Lung Cellular and Molecular Physiology, 313(6), L1101–L1153. https://doi.org/10.1152/ajplung.00343.2017.
McGowan, E. C., Kostadinov, S., McLean, K., Gotsch, F., Venturini, D., & Romero, R., et al. (2009). Placental IL-10 dysregulation and association with bronchopulmonary dysplasia risk. Pediatrics Research, 66(4), 455–460. https://doi.org/10.1203/PDR.0b013e3181b3b0fa.
Li, J., Wang, Z., Chu, Q., Jiang, K., Li, J., & Tang, N. (2018). The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Developmental Cell, 44(3), 297–312. https://doi.org/10.1016/j.devcel.2018.01.008.
Hou, A., Fu, J., Shi, Y., Qiao, L., Li, J., & Xing, Y. et al.(2018). Decreased ZONAB expression promotes excessive transdifferentiation of alveolar epithelial cells in hyperoxia-induced bronchopulmonary dysplasia. International Journal of Molecular Medicine, 41(4), 2339–2349. https://doi.org/10.3892/ijmm.2018.3413.
Ma, Q., Deng, X., Jin, B., Zhang, Y., Luo, D., & Song, H., et al. (2015). A novel human interleukin-24 peptide created by computer-guided design contributes to suppression of proliferation in esophageal squamous cell carcinoma Eca-109 cells. Oncology Reports, 33(1), 193–200. https://doi.org/10.3892/or.2014.3589.
Li, Z., Jiang, W., Wu, G., Ju, X., Wang, Y., & Liu, W. (2018). miR-16 inhibits hyperoxia-induced cell apoptosis in human alveolar epithelial cells. Molecular Medicine Reports, 17(4), 5950–5957. https://doi.org/10.3892/mmr.2018.8636.
Sun, J., Zheng, S., Yang, N., Chen, B., He, G., & Zhu, T. (2019). Dexmedetomidine inhibits apoptosis and expression of COX-2 induced by lipopolysaccharide in primary human alveolar epithelial type 2 cells. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2019.07.023.
Bahmed, K., Lin, C. R., Simborio, H., Karim, L., Aksoy, M., & Kelsen, S., et al. (2019). The role of DJ-1 in human primary alveolar type II cell injury induced by e-cigarette aerosol. American Journal of Physiology—Lung Cellular and Molecular Physiology, https://doi.org/10.1152/ajplung.00567.2018.
Luo, Y. H., Kuo, Y. C., Tsai, M. H., Ho, C. C., Tsai, H. T., & Hsu, C. Y., et al. (2017). Interleukin-24 as a target cytokine of environmental aryl hydrocarbon receptor agonist exposure in the lung. Toxicology and Applied Pharmacology, 324(1-11. https://doi.org/10.1016/j.taap.2017.03.019.
Chen, X., Liu, D., Wang, J., Su, Q., Zhou, P., & Liu, J., et al. (2014). Suppression effect of recombinant adenovirus vector containing hIL-24 on Hep-2 laryngeal carcinoma cells. Oncology Letters, 7(3), 771–777. https://doi.org/10.3892/ol.2014.1789.
Fan, S., Gao, H., Ji, W., Zhu, F., Sun, L., & Liu, Y., et al. (2019). Umbilical cord-derived mesenchymal stromal/stem cells expressing IL-24 induce apoptosis in gliomas. Journal of Cell Physiology, https://doi.org/10.1002/jcp.29095.
Li, Y., Zhang, H., Zhu, X., Feng, D., Gong, J., & Han, T. (2013). Interleukin-24 induces neuroblastoma SH-SY5Y cell differentiation, growth inhibition, and apoptosis by promoting ROS production. Journal of Interferon & Cytokine Research, 33(11), 709–714. https://doi.org/10.1089/jir.2013.0004.
He, M., & Liang, P. (2010). IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. Journal of Immunology, 184(4), 1793–1798. https://doi.org/10.4049/jimmunol.0901829.
Chen, H., Liu, C., Chen, C., Su, Z., Shu, J., & Zhang, M., et al. (2019). Bone morphogenetic protein 4 regulates immortalized chicken preadipocyte proliferation by promoting G1/S cell cycle progression. FEBS Open Bio, 9(6), 1109–1118. https://doi.org/10.1002/2211-5463.12640.
Wang, Y., & Bai, L. (2019). Resveratrol inhibits apoptosis by increase in the proportion of chondrocytes in the S phase of cell cycle in articular cartilage of ACLT plus Mmx rats. Saudi Journal of Biological Sciences, 26(4), 839–844. https://doi.org/10.1016/j.sjbs.2017.04.010.
Jiang, Z., Chen, Z., Li, L., Zhou, W., & Zhu, L. (2017). Lack of SOCS3 increases LPS-induced murine acute lung injury through modulation of Ly6C(+) macrophages. Respiratory Research, 18(1), 217 https://doi.org/10.1186/s12931-017-0707-6.
Duan, W. N., Xia, Z. Y., Liu, M., Sun, Q., Lei, S. Q., & Wu, X. J., et al. (2017). Protective effects of SOCS3 overexpression in high glucoseinduced lung epithelial cell injury through the JAK2/STAT3 pathway. Molecular Medicine Reports, 16(3), 2668–2674. https://doi.org/10.3892/mmr.2017.6941.
Sun, D., Wang, J., Yang, N., & Ma, H. (2016). Matrine suppresses airway inflammation by downregulating SOCS3 expression via inhibition of NF-kappaB signaling in airway epithelial cells and asthmatic mice. Biochemical and Biophysical Research Communications, 477(1), 83–90. https://doi.org/10.1016/j.bbrc.2016.06.024.
Will, J. P., Hirani, D., Thielen, F., Klein, F., Vohlen, C., & Dinger, K. et al.(2019). Strain-dependent effects on lung structure, matrix remodeling, and Stat3/Smad2 signaling in C57BL/6N and C57BL/6J mice after neonatal hyperoxia. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 317(1), R169–R181. https://doi.org/10.1152/ajpregu.00286.2018.
Acknowledgements
This work was financially supported by the key scientific and technological project of the Chinese Ministry of Education (grant no. 212103). The experiment of the expression of IL-24 in lungs exposed to hyperoxia and FATIICs exposed to oxygen and LPS was conducted at the Children’s Hospital of Fudan University. The experiment on the role of IL-24 in FATIIC development was conducted in Binzhou Medical University Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, R., Li, Z., Ai, D. et al. Interleukin-24 as a Pulmonary Target Cytokine in Bronchopulmonary Dysplasia. Cell Biochem Biophys 79, 311–320 (2021). https://doi.org/10.1007/s12013-021-00968-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-00968-z