Abstract
We report experimental speeds of sound in methanol. Measurements were conducted at temperatures from 233 to 353 K with pressures up to 20 MPa using the double-path length pulse-echo technique. The relative expanded combined uncertainty (k = 2) in measurement was estimated to vary from 0.012 to 0.014%, considering contributions from temperature, pressure, path length calibration, pulse timing, and purity of the sample. Experimental speeds of sound gained in the scope of this work were compared with the equation of state by de Reuck and Craven, as well as with further data from literature.
Similar content being viewed by others
1 Introduction
The power-to-methanol technology is considered more and more important with increasing focus on the challenge of storing energy from volatile renewable sources. In such processes, hydrogen descending from electrolysis operated with excess renewable energy is converted to methanol with CO2 captured from, e.g., industrial combustion gases [1,2,3]. At times of insufficient supply of power from renewable sources, stored liquid methanol can be used as fuel to cover the excess demand of electricity. Design and engineering of such processes employ thermophysical property data gained from fundamental equations of state. The currently used fundamental equation of state of methanol was developed by de Reuck and Craven [4]. Since some shortcomings of this equation of state are well known, Thol and Span [5] currently work on a new equation of state. Since such approaches are empirical models, their accuracy is directly related to the quality of the employed experimental data.
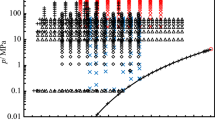
Even though several experimental (p, c, T) data sets of methanol are available in the literature, only very few authors provide comprehensive data sets over larger ranges of temperature and especially pressure (see Table 1). In addition, not all of the available data are consistent, which necessitated further speed of sound measurements to clarify the existing data situation. Temperatures of the isotherms studied in this work have been selected partly to allow for direct comparison with other data sets. In a (p,T) diagram, Fig. 1 shows the state points of speed of sound measurements carried out in the scope of this work as well as of further data available from literature.
Selection of experimental (p, c, T) data of methanol from literature and (p, T) state points investigated in the present work shown in a (p,T) phase diagram.  , this work; ▿, Javed et al. [6]; ◊, Davila et al. [7]; ×, further data from literature; ―, phase boundary and ☆, critical point as calculated with the equation of state of de Reuck and Craven [4] (Color figure online)
, this work; ▿, Javed et al. [6]; ◊, Davila et al. [7]; ×, further data from literature; ―, phase boundary and ☆, critical point as calculated with the equation of state of de Reuck and Craven [4] (Color figure online)
2 Experimental Section
2.1 Apparatus Description
Speed of sound measurements in methanol were conducted employing the double-path length pulse-echo technique with an apparatus according to the design of Meier and Kabelac [58]. The experimental set-up has been used for speed of sound measurements of several liquids in our previous works [59,60,61,62]; thus, it is only explained in brief at this point. A detailed description with illustrations of the measuring cell and a schematic drawing of the entire measuring system can be taken from the work of Gedanitz et al. [63]. The actual acoustic sensor consists of an x-cut quartz crystal as transducer, located axially off-center between two polished stainless steel reflectors, resulting in two different path lengths (L1 = 20 mm and L2 = 30 mm). If the piezoelectric disc transducer is excited by a tone burst (here a 30-cycle sinusoidal burst at 8 MHz) of a waveform generator (Agilent, model 3322A), ultrasonic pulses propagate into the surrounding fluid and are reflected at both ends of the measuring cell. The returning wave fronts excite the transducer and the resulting electronic signals are captured by a digital oscilloscope (Agilent, model MS6032A). These signals are referred to as echoes. Due to the unequal path lengths, the second echo is detected delayed by the time difference ∆techo. The speed of sound can be determined according to Eq. (1), incorporating a correction τ according to Harris [64] to account for diffraction:
The waveform of the two resulting echoes is averaged from 16 consecutive pulses and a bandpass filter based on a Fast Fourier Transform with a bandwidth of 1.6 MHz is applied, to enhance the signal-to-noise ratio. Based on these processed data, the time difference ∆techo is computed according to the algorithm described by Dubberke et al. [65].
The acoustic sensor was housed in a stainless steel pressure vessel and allows for speed of sound measurements up to 20 MPa. Two vibrating-quartz crystal pressure transducers (Paroscientific, models 1000-500A and 1000-6 K) and a differential pressure indicator (Rosemount, model 3051) are utilized to measure the pressure at a standard uncertainty of u(p) = 0.0024 MPa. The entire pressure vessel is immersed into a calibration bath thermostat (Fluke, model 7060), realizing speed of sound measurements at temperatures from 233 to 353 K. The temperature is measured with a long-stem 25 Ω standard platinum resistance thermometer (SPRT, Rosemount Aerospace, model 162CE), calibrated on ITS-90 and read with a direct current thermometry bridge (Isotech, model TTI-2), stating a standard uncertainty in temperature measurement of u(T) = 0.004 K. With measurements over the stated temperature and pressure range, thermal expansion and pressure deformation can affect the actual path length, which is considered according to Eq. (2):
where ∆L0 is the path length difference at the reference temperature T0 = 293.15 K and the reference pressure p0 = 0.1 MPa. The mean value of the isobaric expansivity α at reference pressure p0 was received from values for the differential thermal expansion of stainless steel 1.4571 according to Eq. (3):
While the coefficients ai for i ≥ 1 are values given by Meier [66] according to Table 2, a0 and the path length difference ΔL0 given in Eq. (2) are determined from calibration measurements with high-purity water. Here, the calibration remains the same as presented in our previous work [60], where measurements at T = 274.15, 278.15, 283.15, 293.15, 303.15, 313.15, 323.15, 333.15, 343.15, and 353.15 K and ambient pressure were conducted. The calibration was evaluated by comparison with the IAPWS-95 equation of state by Wagner and Pruß [67] with the experimental speeds of sound being reported within the uncertainty of the equation of state of 0.005%.
In Eq. (2), β describes the mean compressibility of the cell at temperature T and is determined from material properties [68] according to Eq. (4):
where E is Young’s modulus and ν = 0.3 is Poisson’s ratio with the employed coefficients bi given in Table 2.
2.2 Experimental Material
The samples used in the present work are specified in Table 3. High-purity water with a purity of 99.9997 mol% was employed for the calibration. The purity of the used methanol was found to be 99.9300 mol%, investigated by gas chromatographical analysis, as stated by the supplier. Additionally, a mass fraction of water of less than 0.001%, known from coulometric Karl Fischer titration, was stated. In order to avoid contact with the humidity of ambient air, the methanol sample was transferred to a stainless steel bottle within the dry inert argon atmosphere of a glove box that was manufactured in-house. Prior to use, the sample was degassed by repeated freeze–pump–thaw cycles, as described in our previous works [59,60,61,62].
2.3 Experimental Procedure
The experimental procedure applied to conduct the speed of sound measurements in the scope of this work was adapted from previous works [59, 60]. The stainless steel bottle containing the sample of methanol was attached to the evacuated speed of sound apparatus and carefully heated. Simultaneously, the temperature of the bath thermostat was set to the lowest possible temperature of 233 K, thus taking advantage of the pressure and temperature gradient during the filling process. A connected hand pump was utilized to increase the pressure up to 20 MPa and the system was left to equilibrate prior to the first measurement. Measurements were subsequently carried out along isotherms with decreasing pressure. From isotherm to isotherm, the temperature of the measuring cell was increased overnight.
2.4 Uncertainty Analysis
The combined expanded uncertainty in speed of sound was estimated according to our previous studies [59, 60], where the approach is explained in detail, by developing the following term with consideration of Eqs. (1) and (2):
The square of the standard uncertainty of the speed of sound is then described by Eq. (6):
Further contributions resulting from the measurement of temperature and pressure as well as from the presence of impurities are considered within the determination of the expanded combined uncertainty U(c) according to Eq. (7) with the expansion factor k = 2:
The respective derivatives were determined by sensitivity analysis, employing the equation of state of de Reuck and Craven [4]. The impact of potential impurities was investigated using mixture models, implemented in TREND 4.0 [69]. Water and ethanol were investigated as the impurities with the largest influence. The result for the combined expanded uncertainty U(c) for the speed of sound measurement in methanol at T = 293.20 K and p = 5 MPa is exemplified in Table 4 and was estimated to be 0.134 m·s−1. Among all conducted measurements, U(c) varies between 0.126 and 0.176 m·s−1, while the relative expanded combined uncertainty remains almost constant with values from 0.012 to 0.014%.
3 Results and Discussion
The speed of sound was measured at seven or eight state points on each of nine isotherms, leading to a (p, c, T) data set comprising 65 measuring points (see Table 5). Absolute experimental speeds of sound are plotted versus pressure in Fig. 2, clarifying internal consistency throughout the entire (p,T) region and showing a decreasing speed of sound with increasing temperature. In the present work, speeds of sound in methanol vary from 926 m·s−1 (T = 353 K and p = 0.25 MPa) to 1420 m·s−1 (T = 233 K and p = 20 MPa).
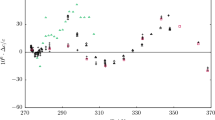
Relative deviations of the experimental speeds of sound in methanol from values calculated with the equation of state of de Reuck and Craven [4] as reference (zero line) are plotted versus pressure in Fig. 3. Relative deviations of the entire data set fall well within the uncertainty of the equation of state, stated by the authors to be 2%. However, a trend can be observed along each isotherm with the relative deviations from the lowest to the highest pressure decreasing between 0.27 and 0.42%. Altogether, relative deviations vary from –0.66% at T = 353 K and p = 15.03 MPa to 0.35% at T = 253 K and p = 0.16 MPa.
Results of the speed of sound measurements in methanol. Relative deviations of the experimental speeds of sound cexp from values cEOS calculated with the equation of state of de Reuck and Craven [4] are plotted versus pressure.  , 233 K;
, 233 K;  , 253 K;
, 253 K;  , 273 K;
, 273 K;  , 293 K;
, 293 K;  , 300 K;
, 300 K;  , 313 K;
, 313 K;  , 333 K;
, 333 K;  , 350 K;
, 350 K;  , 353 (Color figure online)
, 353 (Color figure online)
Again, Fig. 4 shows the comparison of the speed of sound measured in the scope of this work with the equation of de Reuck and Craven [4] as reference (zero line). However, the comparison focuses on the individual temperatures and can thus consider further data sets from literature. The data set by Davila et al. [7] is in very good agreement with our reported data and falls within the experimental uncertainty at temperatures between 273 and 333 K. At 253 and 353 K both data sets differ by up to 0.04%, slightly exceeding the claimed combined experimental uncertainty. Speeds of sound, gained in the scope of this work, are also in good agreement with the data set by Javed et al. [6] at 350 K, especially at higher pressures, where deviations are within the experimental uncertainty. Comparison with data from Javed et al. [6] at lower temperatures shows a systematical downward offset at 300 K and a systematical upward offset at 244 and 253 K, respectively (see Fig. 4). Further data by Sun et al. [54] and Wilson and Bradley [55] show a upward offset at overlapping temperatures from 273 to 313 or 333 K and at comparable pressures (see Fig. 4).
Results of the speed of sound measurements of methanol. Relative deviations of the experimental speeds of sound cexp from values cEOS calculated with the equation of state by de Reuck and Crave [4] (zero line) are plotted versus pressure.  , this work; ◊, Davila et al. [7]; ▿, Javed et al. [6]; ◻, Sun et al. [54]; ○, Wilson and Bradley [55] (Color figure online)
, this work; ◊, Davila et al. [7]; ▿, Javed et al. [6]; ◻, Sun et al. [54]; ○, Wilson and Bradley [55] (Color figure online)
4 Conclusion
Speeds of sound in high-purity methanol were measured at temperatures from 233 to 353 K and at pressures up to 20 MPa with the overall relative expanded combined uncertainty (k = 2) ranging from 0.012 to 0.014%. The new data deviate from the equation of state of de Reuck and Craven [4] by up to 0.66% but agree with the data set of Davila et al. [7] within 0.014% at temperatures between 273 and 333 K and within 0.040% beyond this temperature range. The measurements conducted by Javed et al. [6] are confirmed partly, especially at higher temperatures. On other isotherms significant deviations to the data of Javed et al. [6] are observed.
References
M. Nielsen, E. Alberico, W. Baumann, H.-J. Drexler, H. Junge, S. Gladiali, M. Beller, Nature (2013). https://doi.org/10.1038/nature11891
M.J. Bos, S.R.A. Kersten, D.W.F. Brilman, Appl. Energy (2020). https://doi.org/10.1016/j.apenergy.2020.114672
I. González-Aparicio, Z. Kapetaki, E. Tzimas, Appl. Energy (2018). https://doi.org/10.1016/j.apenergy.2018.03.114
K.M.d. Reuck, R.J.B. Craven, S. Angus, B. Armstrong, R.J. Craven, W. Wagner, Methanol - International Thermodynamic Tables of Fluid State Vol. 12 (Blackwell Scientific Publications, London, 1993)
M. Thol, R. Span, Int. J. Thermophys. (to be submitted 2021)
M.A. Javed, E. Baumhögger, J. Vrabec, J. Chem. Eng. Data (2019). https://doi.org/10.1021/acs.jced.8b00938
M.J. Dávila, H. Gedanitz, R. Span, J. Chem. Thermodyn. (2016). https://doi.org/10.1016/j.jct.2015.10.006
R. Abraham, M. Abdulkadhar, C.V. Asokan, J. Chem. Thermodyn. (2000). https://doi.org/10.1006/jcht.1999.0608
R. Abraham, M. Abdulkadhar, C.V. Asokan, Acoust. Lett. 20, 236–245 (1997)
D.S. Allam, W.H. Lee, J. Chem. Soc. A (1966). https://doi.org/10.1039/J19660000005
T.M. Aminabhavi, M.I. Aralaguppi, S.B. Harogoppad, R.H. Balundgi, J. Chem. Eng. Data (1993). https://doi.org/10.1021/je00009a008
T.M. Aminabhavi, K. Banerjee, J. Chem. Eng. Data 43, 1096 (1998)
T.M. Aminabhavi, V.B. Patil, K. Banerjee, J. Chem. Eng. Data (1999). https://doi.org/10.1021/je990120q
A. Arce, O. Rodrı́guez, A. Soto, Fluid Phase Equilib. (2004) doi:https://doi.org/10.1016/j.fluid.2004.05.008
A. Arce, E. Rodil, A. Soto, J. Solution Chem. (2006). https://doi.org/10.1007/s10953-006-8939-y
A.I. Artemchenko, Zh. Fiz, Khim 37, 3 (1963)
I. Bahadur, N. Deenadayalu, J. Solution Chem. (2011). https://doi.org/10.1007/s10953-011-9740-0
M.S. Bakshi, J. Singh, H. Kaur, S.T. Ahmad, G. Kaur, J. Chem. Eng. Data (1996). https://doi.org/10.1021/je960180l
H.B. Bohidar, J. Appl. Phys. (1988). https://doi.org/10.1063/1.341780
E.H. Carnevale, T.A. Litovitz, J. Acoust. Soc. Am. (1955). https://doi.org/10.1121/1.1907959
L.M. Casás, G. Marino, M. Iglesias, B. Orge, J. Tojo, L. Mosteiro, J. Chem. Thermodyn. (2001). https://doi.org/10.1006/jcht.2001.0878
M.S. Chauhan, K.C. Sharma, S. Gupta, Acoust. Lett. 18, 233–240 (1995)
F. Danusso, E. Fadigati, Atti Accad. Naz. Lincei 14, 81 (1953)
M.J. Dávila, J.P.M. Trusler, J. Chem. Thermodyn. (2009). https://doi.org/10.1016/j.jct.2008.08.003
H.F. Eden, Richardson E. G., Acta Acust. united Acust. 10, 309 (1960)
J. Emery, S. Gasse, Acta Acust. united Acust. 43, 205 (1979)
R.J. Fort, W.R. Moore, Trans. Faraday Soc. (1965). https://doi.org/10.1039/TF9656102102
E.B. Freyer, J.C. Hubbard, D.H. Andrews, J. Am. Chem. Soc. (1929). https://doi.org/10.1021/ja01378a014
S.V.S. Gadaibaev, M.I. Shakhparonov, Moscow Univ. Chem. Bull. 28, 66 (1973)
J. George, N.V. Sastry, S.R. Patel, M.K. Valand, J. Chem. Eng. Data (2002). https://doi.org/10.1021/je010268l
D.S. Gill, J. Singh, R. Ludwig, M.D. Zeidler, J. Chem. Soc., Faraday Trans. (1993) doi:https://doi.org/10.1039/FT9938903955
A.Z. Golik, Y.I. Shimanskii, N.M. Kobiichuk, Ukr. Fiz. Zh 3, 537 (1958)
B. González, A. Domínguez, J. Tojo, J. Chem. Eng. Data (2004). https://doi.org/10.1021/je0342825
S. Govindarajan, V. Kannappan, M.D. Naresh, K. Venkataboopathy, B. Lokanadam, J. Mol. Liq. (2003). https://doi.org/10.1016/S0167-7322(03)00156-9
M. Iglesias, B. Orge, M. Domínguez, J. Tojo, Phys. Chem. Liq. (1998). https://doi.org/10.1080/00319109808032796
M. Iglesias, B. Orge, M.M. Piñeiro, B.E. de Cominges, G. Marino, J. Tojo, J. Chem. Eng. Data (1998). https://doi.org/10.1021/je980041z
H. Iloukhani, Z. Rostami, J. Solution Chem. (2003). https://doi.org/10.1023/A:1024524928767
S.K.K. Jatkar, J. Indian I. Sci. Sec. A-Sci. 22, 19 (1939)
A. Kumar, O. Prakash, S. Prakash, J. Chem. Eng. Data (1981). https://doi.org/10.1021/je00023a021
G. Marino, M. Iglesias, B. Orge, J. Tojo, M.M. Piñeiro, J. Chem. Thermodyn. (2000). https://doi.org/10.1006/jcht.1999.0615
A. Moses Ezhil Raj, L.B. Resmi, V.B. Jothy, M. Jayachandran, C. Sanjeeviraja, Fluid Phase Equilib. (2009) doi:https://doi.org/10.1016/j.fluid.2009.04.009
H. Ogawa, S. Murakami, J. Solution Chem. 16, 315 (1987)
T. Ohmori, Y. Kimura, N. Hirota, M. Terazima, Phys. Chem. Chem. Phys. (2001). https://doi.org/10.1039/b101773j
B. Orge, M. Iglesias, A. Rodríguez, J.M. Canosa, J. Tojo, Fluid Phase Equilib. (1997). https://doi.org/10.1016/S0378-3812(97)00031-9
S. Parthasarathy, A.F. Chhapgar, H. Singh, Nuovo Cimento (1953). https://doi.org/10.1007/BF02786196
S.M. Pereira, M.A. Rivas, T.P. Iglesias, J. Chem. Eng. Data (2002). https://doi.org/10.1021/je020028l
S.M. Pereira, M.A. Rivas, J.L. Legido, T.P. Iglesias, J. Chem. Thermodyn. (2003). https://doi.org/10.1016/S0021-9614(02)00311-7
M.J.W. Povey, S.A. Hindle, J.D. Kennedy, Z. Stec, R.G. Taylor, Phys. Chem. Chem. Phys. (2003). https://doi.org/10.1039/B206425A
I.B. Rabinovich, V.G. Golov, N.A. Efimova, S.M. Rustamov, Dokl. Akad. Nauk 114, 590 (1957)
M. Rama Rao, Indian J. Phys. 14, 109 (1940)
G.V.R. Rao, P.B.S. Sri, A.V. Sarma, C. Rambabu, Ind. J. Pure Appl. Phys. 45, 135 (2007)
J.M. Resa, C. González, M.A. Fanega, S. Ortiz de Landaluce, J. Lanz, J. Food Eng. (2002). https://doi.org/10.1016/S0260-8774(01)00045-0
A. Rodríguez, J. Canosa, J. Tojo, J. Chem. Eng. Data (2001). https://doi.org/10.1021/je010148d
T. Sun, S.N. Biswas, N.J. Trappeniers, A. ten Seldam, J. Chem. Eng. Data (1988). https://doi.org/10.1021/je00054a002
W. Wilson, D. Bradley, J. Acoust. Soc. Am. (1964). https://doi.org/10.1121/1.1918956
Z. Yu, H. Gao, H. Wang, L. Chen, J. Chem. Eng. Data (2011). https://doi.org/10.1021/je2000606
M.T. Zafarani-Moattar, H. Shekaari, J. Chem. Eng. Data (2005). https://doi.org/10.1021/je050165t
K. Meier, S. Kabelac, Rev. Sci. Instrum. (2006). https://doi.org/10.1063/1.2400019
C.W. Scholz, M. Richter, Int. J. Thermophys. (2021). https://doi.org/10.1007/s10765-020-02778-6
C.W. Scholz, Y. Sanchez-Vicente, T. Tananilgul, M. Thol, J.P.M. Trusler, M. Richter, J. Chem. Eng. Data (2020). https://doi.org/10.1021/acs.jced.0c00299
R. Wegge, M. Richter, R. Span, J. Chem. Eng. Data (2015). https://doi.org/10.1021/je501065g
R. Wegge, M. Richter, R. Span, Fluid Phase Equilib. (2016). https://doi.org/10.1016/j.fluid.2015.10.034
H. Gedanitz, M.J. Dávila, E. Baumhögger, R. Span, J. Chem. Thermodyn. (2010). https://doi.org/10.1016/j.jct.2009.11.002
G.R. Harris, J. Acoust. Soc. Am. (1981). https://doi.org/10.1121/1.386671
F.H. Dubberke, E. Baumhögger, J. Vrabec, Rev. Sci. Instrum. (2015). https://doi.org/10.1063/1.4921478
K. Meier, Dissertation, Helmut-Schmidt-Universität - Universität der Bundeswehr Hamburg, 2006, https://www.hsu-hh.de/thermodynamik/wp-content/uploads/sites/741/2017/09/Habilitationsschrift-Meier.pdf. Accessed 8 May 2020
W. Wagner, A. Pruß, J. Phys. Chem. Ref. Data (2002). https://doi.org/10.1063/1.1461829
Verein Deutscher Eisenhüttenleute, Stahl-Eisen-Werkstoffblätter (1997)
R. Span, R. Beckmüller, T. Eckermann, S. Herrig, S. Hielscher, A. Jäger, T. Neumann, S. Pohl, B. Semrau, M. Thol, TREND. Thermodynamic Reference and Engineering Data (Lehrstuhl für Thermodynamik: Ruhr-Universität Bochum, Bochum, 2019)
Acknowledgements
The authors thank Dr. Monika Thol of RUB for helpful discussions regarding the present measurements.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scholz, C.W., Span, R. Speeds of Sound in Methanol at Temperatures from 233.33 to 353.21 K at Pressures up to 20 MPa. Int J Thermophys 42, 65 (2021). https://doi.org/10.1007/s10765-021-02811-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-021-02811-2