Abstract
Soft tissue engineering has been gaining increasing interest as an approach to overcome the limitations posed by current clinical procedures such as invasiveness of the surgery, post-operative complications and volume loss. Soft tissue damage occurs either due to congenital malformation, trauma/disease or surgical resection. Through the use of autologous cells, such as mesenchymal stem cells, combined with a biomaterial acting as a support, biological substitutes can be developed. A promising pathway in terms of delivery of these engineered constructs is the use of an injectable system, able to provide a minimally invasive approach. Advances have been made in the development of biocompatible biomaterials able to induce soft tissue regeneration. The present review provides an overview of fillers used in the clinic as well as a non-exhaustive overview of all injectable systems reported for soft tissue engineering. A particular focus is placed on the benefits and drawbacks of the biomaterials and the underlying polymerisation strategy. Furthermore, focus is placed on the mechanical properties of the systems.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Soft tissue engineering covers a broad spectrum of tissues such as fat, fibrous tissue, brain, muscle and skin. Due to congenital defects, disease or trauma, soft tissue damage can occur. Autologous tissue has often been used in clinics as a method to restore the damaged tissue, however, several drawbacks, such as lengthy and invasive surgeries, cell absorption and volume loss as well as post-operative complications have limited its use [1, 2]. Therefore, tissue engineering has been gaining increasing interest as an approach to overcome the barriers posed by current clinical approaches as new biological substitutes can be applied [3].
Tissue engineering (TE) aims to replace or restore the damaged sites, overcoming aesthetical disturbances and targeting functional restoration [4]. In this respect, living cells can be expanded in vivo and combined with a scaffold or support system that can act as an artificial extracellular matrix (ECM). The ECM mimic should degrade over time, allowing the cells to proliferate and secrete their own ECM until the tissue is fully regenerated, as can be seen in figure 1.
Figure 1. An overview of tissue regeneration starting from the hydrogel precursors to the regenerated tissue.
Download figure:
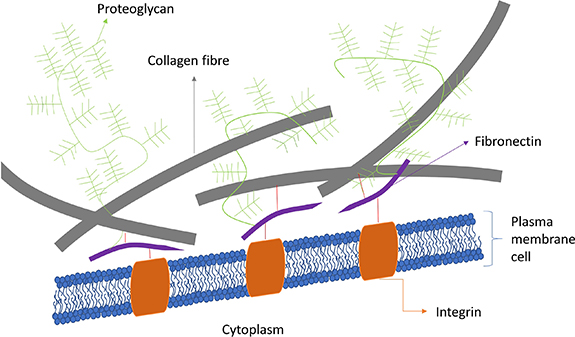
Standard image High-resolution imageIn literature, focus has been placed on developing a mimic of the native ECM in terms of structure and function. Understanding the role of the ECM and all its cues is important to grasp its potential and complexity. The ECM is a highly organized three-dimensional system offering mechanical integrity, providing structural support and maintaining normal tissue architecture. Furthermore, it is a dynamic system in which signalling cues such as cytokines and growth factors can modulate cell–matrix and cell–cell interactions, i.e. so-called intercellular crosstalk. These biochemical and physical cues as well as mechano-sensing cues can control cell behaviour and fate. Furthermore, there is a direct relation between the ECM and the cells due to cell receptors, called integrins, which will react with certain ECM components, such as the tripeptide Arg-Gly-Asp (RGD) [5, 6]. Due to the presence of a vast number of matrix metalloproteinases (MMPs) acting as digestive enzymes, the ECM is continuously remodelled. Structurally, the ECM consists of several macromolecules such as collagen type-I, -II, -III, -V, and -XI, fibronectin, laminin, vitronectin and elastin, which are present in different ratios depending on the localisation [7, 8]. An overview of a basic ECM structure can be seen in figure 2.
Figure 2. An overview of the ECM composition. The cell membrane is depicted with integrins connected to fibronectin. The fibronectin in its turn is attached to both collagen fibres as well as proteoglycans.
Download figure:
Standard image High-resolution imageAs the material will be implanted and surrounded by human tissue, it is of importance that the material is compatible with living tissue. Biocompatibility is defined as a compound being non-toxic or injurious to a living system. Furthermore, the material may not cause immunological rejection and needs to be able to support a cell–biomaterial interaction [9, 10]. A polymer is regarded to be biocompatible only when it will not cause an inflammatory reaction which is not proportionate to its benefits. It thus must degrade at the implant site into safe degradation products that will, in the end be fully eliminated from the body, without any traces being left. Furthermore, it needs to be free from toxic compounds, pyrogens and any other microbial agents [11].
Thus, the biomaterials need to mimic the ECM to acquire an optimal environment for stem cell proliferation and differentiation [12]. They can be constituted by either naturally derived or synthetic polymers, each entailing their own advantages as well as disadvantages. Collagen, gelatin and hyaluronic acid (HA) will result in superior cell attachment and cell proliferation compared to synthetic polymers such as polylactic acid (PLA), polyethylene glycol (PEG) or polyethylene terephthalate [13, 14]. While biopolymer-based hydrogels offer the advantage of bioactivity, they often lack control over their mechanical properties in contrast with synthetic materials [14].
Furthermore, minimally invasive delivery of these biomaterials, as can be seen in figure 3, is vital to omit any need for open surgery, thereby increasing patient comfort and reducing morbidity and expenses [15]. An injectable biomaterial can function as a support for cells. The focus of the biomaterial should not only be to repair the lost/damaged tissue, but furthermore, it needs to induce the natural regeneration potential of the cells present. In the end, the biomaterial should be integrated within the surrounding tissue, as illustrated in figure 3. One of the critical aspects of an injectable system is its viscosity as this will directly impact the ease of injection [14, 16]. Therefore, mostly precursor solutions have been used that will crosslink and thus solidify in situ via numerous crosslinking techniques including both chemical (Michael addition, Schiff-base, redox) and/or physical (ionic, pH, temperature) [17].
Figure 3. Minimally invasive approach through an injectable system. A defect site can be targeted via injection through a needle. Both biomaterials and autologous cells will be deposited, followed by cell proliferation, ECM secretion and material degradation.
Download figure:
Standard image High-resolution imageTo date, injectable biomaterials are only omnipresent in the aesthetical market. However, these systems are mostly prone to rapid resorption. These injectables find their use, not only for elective aesthetical surgeries, but also in reconstructive procedures. They can potentially target congenital defects, trauma and surgical resections focussing on regaining and regenerating the lost and/or damaged tissue, such as loss of subcutaneous fat, burn wound scarring or resections due to f.e. breast cancer [18, 19]. Furthermore, the injectable systems could also function as a drug and/or cell delivery platform.
Herein, the state of the art will be assessed to cover both the advantages and disadvantages associated with injectable systems in soft tissue reconstruction and tissue engineering.
2. Naturally derived polymers
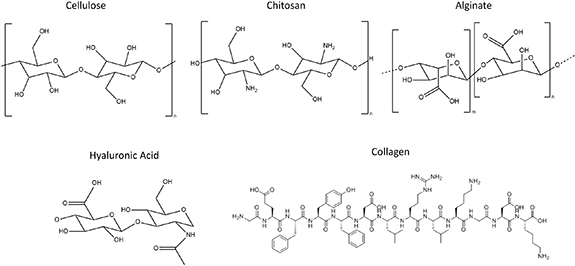
Biomaterials can either be naturally derived or synthetic. Naturally derived materials, such as gelatin, HA, alginate, chitosan or collagen possess more innate biological functions, compared to synthetic polymers. They are however prone to rapid degradation and exhibit a low mechanical strength. These disadvantages can be tackled by the introduction of crosslinkable functionalities along the backbone. An overview of the chemical structures can be found in figure 4.
Figure 4. An overview of the chemical structure of some naturally derived polymers.
Download figure:
Standard image High-resolution imageCellulose is the most abundantly available natural polymer, which can be found mainly in plants and micro-organisms. Thus, the material can be easily sourced and offer a low cost platform [20]. It is insoluble and non-degradable in a physiological environment, meaning that new tissue will not occupy the space where cellulose is present. Cellulose is an organic polysaccharide that exhibits hydrophilic behaviour [21, 22]. It has already found its entry in drug and cell delivery systems. Schaschkow et al have described a hydroxypropylmethyl cellulose hydrogel, which can be used for an islet transplantation system [23]. Furthermore, the mechanical properties of cellulose will differ based on the material source f.e. bacterial vs plant-based. Therefore, depending on the biomedical application, the desired mechanical properties can be selected [24, 25]. However, these mechanical properties range between 250 MPa and 15 GPa for bacterial-derived cellulose and are thus less ideal for soft tissue engineering [25].
Chitosan, also described as poly-D-glucosamine, is obtained by deacetylation of chitin. It is a viscous, semi-crystalline material, which is insoluble in water [26]. A phenolic compound-conjugated chitosan injectable has already been assessed to serve as a sustained therapeutic drug delivery platform for glaucoma treatment [27]. Furthermore, a glycol-chitosan network has also been described of being able to enhance the cell proliferation rate of human dental pulp cells which also exhibited superior odontoblastic differentiation and mineralization potential [28]. One of the main advantages of chitosan is that all degradation products are biocompatible and will not cause any inflammatory effect. It is the second most abundant polysaccharide and thus also offers a low cost platform [29]. Furthermore, it has been shown to be anti-tumoral as well as both wound healing and haemostatic [30, 31]. However, chitosan itself is not cell-interactive.
Alginate is a hydrophilic polysaccharide, which is isolated from algae [32]. It consists of alternating repeated residues of D-mannuronate and L-gluronate. The mechanical properties of alginate will depend on the ratio of these repeating units along with its molecular weight. An injectable alginate and calcium-crosslinked gel with encapsulated primary chondrocytes has been shown to effectively regenerate cartilage in vivo [33]. Alginate can also be seen as another low cost platform due to its high availability. Furthermore, alginate is an FDA approved material thereby facilitating clinical translation [34]. However, alginate is more difficult to degrade due to the high molar masses and will most likely not reach the renal clearance threshold of 50 kDa [35].
Furthermore, macromolecules present in the ECM have also been used as starting material. For example, HA is a polysaccharide, found as a key component of the ECM and synthesized at the cell surface. It mostly is characterized by a high molecular weight and a high dispersity, varying between 1000 and 8000 kDa and consists of alternating N-acetyl-D-glucosamine and D-glucuronic acid residues that are linked [36, 37]. HA is also known to have a high turnover rate in the body as the ECM is constantly renewed. In aesthetics, HA-based fillers are widely used, mostly to improve skin contouring and skin depressions. HA has limited immunogenicity, is biodegradable and contributes to both cell proliferation and migration offering a good starting material for biomedical applications [38]. Currently, HA is mostly produced through microbial fermentation such as via streptococcus zooepidemicus or through extraction from animal tissue. However, both methods suffer from low yields increasing the cost of the base material compared to the above-mentioned natural polymers [39, 40].
Collagen, another ECM component, is also often used. However, without modifications these materials are plagued with a limited longevity rendering it a temporary solution due to the presence of MMPs. Collagen is comprised of three helices which are able to self-aggregate to form collagen fibres. Since the material is biodegradable and has a good biocompatibility, it is widely used in biomedical applications. In cardiac TE, an injectable collagen biomaterial has been reported to improve cell survival and furthermore, attenuates cardiac inflammation, remodelling of the left ventricle and hypertrophy of cardiomyocytes in vivo [41]. Gelatin, derived from collagen, is generally preferred as it is less immunogenic and exhibits excellent water solubility [13]. Thi et al have described an injectable, antioxidant gallic acid-conjugated gelatin which was incorporated into gelatin-hydroxyphenyl propionic hydrogels for enhancing wound healing efficacy. The system scavenges radical oxygen species thereby promoting wound healing and tissue regeneration [42].
Platelet rich plasma (PRP) has already been used for the treatment of superficial skin ailments. It is a plasma fraction, consisting of a high platelet concentration (relative to whole blood). PRP is interesting due to the presence of α-granules which will secrete several growth factors upon activation [43]. Furthermore, fibrin, widely used as a sealant in clinical applications, has potential as an injectable system. Platelet-rich fibrin has already found its way into the clinic together with PRP for microneedle therapies and post-resurfacing [44]. A major advantage of PRP is that it involves an autologous, xeno-free approach, thus being non-immunogenic. Nevertheless, large donor variability has already been described and thus the results are unpredictable. Furthermore, it shows more promise as an additive then as a biomaterial [45].
Lastly, decellularized matrices could potentially be used as they still maintain the natural ECM architecture. Matrigel is an example of these decellularized matrices, extracted from Englebreth-Holm-Swarm tumours and is widely used in research. The advantage of matrigel is the fact that several growth factors and cytokines are still present [46, 47]. However, as the material is derived from decellularized cancerous tissue, clinical translation will be limited. Glyaderm, a decellularized skin matrix has already found to be a good alternative as allograft for burn wound treatment. The acellular dermis consists of both collagen and elastin fibres [48]. Research is currently ongoing on the use of decellularized adipose tissue and its regenerative potential [49, 50].
3. Synthetic polymers
Mechanical properties are an important aspect in order to design the ideal soft tissue filler. Synthetic polymers can easily be tailored towards specific chemical and physical properties. However, they are suboptimal concerning conditions associated with cell proliferation due to their biological inertness [ 14 ]. An overview of the discussed synthetic polymers can be found in figure 5.
Figure 5. An overview of the chemical structures of the synthetic polymers discussed.
Download figure:
Standard image High-resolution imagePoly(ethylene oxide) (PEO), is an FDA-approved, synthetic polymer used for several medical applications. It can be photo-crosslinked with a suitable photo-initiator following modification and exhibits hydrophilic properties. In cartilage TE, an injectable system in the presence of chondrocytes has been described by Sims et al [51]. PEO-b-poly(propylene oxide)-b-PEO (PEO-PPO-PEO), also known as pluronics, are thermo-responsive hydrogels, which exhibit a lower critical solution temperature (LCST). Pluronics have already found their use in drug delivery purposes [52]. Since the LCST of these poloxamers is around body temperature (37 °C), cell encapsulation can take place in mild conditions. Furthermore, at room temperature, the material will be easily injected [52].
PEG is widely used in TE applications. Several articles describe an injectable PEG-based system for bone and cartilage tissue engineering [53, 54]. Chondrocyte-laden 4-arm PEG already showed excellent integration as well as chondrogenesis in vivo [53]. Moreover, RGD-modified PEG was assessed as osteoblast encapsulation matrix showing good cell viability and mineral deposits in vitro [54]. One of the advantages of PEG is that it is biodegradable, offering a slow reabsorption allowing for cellular regeneration [55]. The degradation cannot occur through hydrolytic degradation, due to the ether bounds, but through oxidative degradation, resulting in a significant slower degradation compared to f.e. polyesters. Furthermore, the slow reabsorption during in vivo regeneration will depend on the encapsulated cells, thereby limiting its function as an injectable system [56].
Poly(methyl methacrylate) (PMMA) has been applied in bone, craniofacial and dental applications. PMMA will offer a permanent solution as it is not biodegradable. Due to the stiffness, it is more useful to be applied for bone tissue engineering. Therefore, hybrid systems are typically being researched. A PMMA and dextran mixture was used in an in vivo experiment for soft tissue augmentation showing an excellent injectable and biocompatible system [57]. Furthermore, PMMA cement has also been widely used. The cement can provide an injectable hard tissue filler for bone defects. When combined with calcium silicate, the hybrid system provided a biodegradable system that promoted osteogenesis [58].
PLA and polylactic-co-glycolic acid (PLGA) have been proven to be biocompatible and biodegradable. They are both FDA-approved and have found applications in the biomedical world for both drug delivery as well as fixation devices. PLGA microspheres have been described as injectable for (bone) tissue engineering [59]. However, the material is hydrophobic, which might limit its biomedical applications. Furthermore, although biodegradable, PLA and PLGA exhibit slow degradation kinetics, hydrophobicity and high crystallinity. These materials will thus most likely be used for hard tissue repair, such as bone. Furthermore, PLA and PLGA will form acidic by-products upon degradation [60].
Another synthetic material already used in a clinical setting is liquid silicone. Subdermal injection of the material mitigates high pressures in feet resulting from diabetic foot ulcers. Furthermore, studies have shown that silicone particles combined with growth factors such as basic fibroblast growth factor promote fibroblast aggregation [61]. It offers a permanent solution and has been reported to be non-carcinogenic and minimally antigenic. However, severe immune reactions to silicone have already been reported as well as granulomas and oedema, making it a less ideal candidate for tissue engineering [62].
Poly(vinyl alcohol) (PVA), a water-resorbable polymer, has already been described as both a drug and cell delivery system, mostly focussing on bone and cartilage tissue engineering. The microgels obtained can be loaded with human bone-marrow-derived mesenchymal stem cells thereby promoting osteogenic differentiation [63, 64]. It is a biocompatible, biodegradable, flexible and non-toxic material [65, 66]. Furthermore, it is a relatively cheap material [66].
Poly(N-vinylcaprolactam) (PNVCL) is a polymer which offers thermo-responsive properties, namely LCST, which was proven to be biocompatible. It can be tailored towards specific needs for both tissue engineering and drug delivery applications [67]. It is biodegradable and does not produce and toxic degradation products. As mentioned earlier, due to the LCST, cells can be encapsulated under relatively mild conditions and the system is easily injectable at room temperature [68].
Poly(y-glutamic acid) (y-PGA) is a non-toxic, biocompatible material formed by bacterial fermentation. The peptide bonds constituting y-PGA are resulting from the reaction occurring between the amino groups of glutamic acid (GA) and the carboxylic acid groups at the end of the side chain of GA. The material is biodegradable, non-immunogenic and FDA-approved, holding potential for soft tissue engineering applications. However, one of the disadvantages of PGA is the formation of acidic degradation by-products [69]. A hydrogel mixture with PGA and chitosan has already been described to obtain a thermosensitive sol-gel transition at body temperature [70]. Poly(α-glutamic acid), another isomer has also been used in drug delivery systems towards cancer treatment [71].
Lastly, recombinant proteins and peptides have also been described that can potentially overcome the limited availability of certain natural polymers or even obtain completely self-assembled compositions, tailored to the needs of the specific application [72–74]. Furthermore, recombinant proteins can also overcome drawbacks in terms of reproducibility, which often is lacking for natural polymers.
4. Commercially available injectable systems
Most commercial fillers are HA-based, especially in an esthetical environment to improve skin contouring and depressions. However, several other systems exist as illustrated in table 1. The benefit of these fillers is that minimally invasive corrections can be performed through subcutaneous injections.
Table 1. Overview of commercially available fillers.
| Filler | Composition | Application | Advantage | Disadvantage | Ref | |
|---|---|---|---|---|---|---|
| Natural | Radiesse | Calcium hydroxyapatite microspheres | Injectable system mostly used in different facial regions to obtain projection thereby enhancing facial features such as the cheeks, glabellar lines,... | Long-term effects, induces collagen production | Foreign body response, non-degradable, skin discoloration | [75] |
| Macrolane | Stabilized HA (xenofree) | Mostly used for volume restoration and shaping of the breast and buttocks | Decreased immunogenicity, dynamic viscosity, easily degraded via hyaluronidase | Oedema, erythema, allergic reaction | [76] | |
| Juvéderm | Monophasic monodensified gel composed of crosslinked HA | Primarily used as a facial filler for small lines, folds and wrinkles | [77] | |||
| Prevelle Silk | Bacterial-derived HA containing lidocaine | Injectable system targeting mid and deep dermis corrections for facial wrinkles and folds | [78] | |||
| Restylane | Partially crosslinked HA derivative obtained from a bacterial (Streptococcus) fermentation | Mid- to deep dermal injection for wrinkles and folds | [79] | |||
| Captique | HA-based with single crosslinking | Injectable systems applied to fill wrinkles and skin sag | [80] | |||
| Puragen | HA-based with double crosslinking | Increased longevity | ||||
| Synthetic | Lipen-10 | A mixture of PMMA and crosslinked dextran | Lipen-10 is used for soft tissue augmentation such as penile augmentation and dermal filling | Permanent | Foreign body reaction, non-degradable | [81] |
| Sculptra | Irregularly shaped poly-L-lactic acid (PLLA) microspheres | FDA-approved for facial volume replacement in the treatment of lipoatrophy | Stimulate fibroblasts to make collagen and elastic fibers; longer results, biodegradable | Limited correction, nodules | [18] | |
| Arthecoll | A gel suspension of 20% polymethyl methacrylate (PMMA) microspheres and 3.5% bovine collagen | Permanent soft tissue dermal augmentation | Permanent solution | Foreign body reaction, non-degradable, animal-derived collagen, possibility of displacement, nodules | [82] | |
| Ellansé | Microspheres of a bioresorbable poly-ε-caprolactone in an aqueous carboxymethyl cellulose gel carrier | Ellansé is mostly targeting aesthetical facial injection for cosmetical filling of wrinkles, folds focussing on more long-lasting results | Biodegradable | [83] | ||
| Aquamid | A polyacrylamide hydrogel | Permanent filler to enhance facial soft tissue volume | Integrates with surrounding tissue | Foreign body reaction, non-degradable | [84] | |
| Bio-Alcamid | Poly(alkylimide) | Fat transfer alternative, jaw and chin augmentation | Longevity and resistance to hydrolysis | Capsule formation, migration, inflammation | [85] | |
As most systems are not human-derived, there is a risk of a foreign body response to occur. For example, Arthecoll being one of the commercially available injectables has been described to cause severe inflammation [86]. Furthermore, due to degradation of the construct, a prolonged immune response can be present causing inflammation and the formation of pro-inflammatory cytokines via macrophages and lymphocytes [87].
Currently available aesthetic commercial systems are classified as temporary solutions due to the need for repeated injections in order to maintain the desired result. Additionally, they also lack the potential to induce tissue regeneration. Therefore, research is currently aiming not only to reduce its degradation rates thereby ensuring volume persistence along with improving the biological performance, but moreover, new strategies promoting tissue regeneration are being developed.
5. Injectable systems for soft tissue regeneration
As mentioned before, current clinically available injectable systems, lack the ability to promote tissue regeneration. Therefore, research has been devoted in developing novel strategies for soft tissue regeneration. One of the main disadvantages of the commercial systems is its temporary nature. Both the mechanical properties and stability of a biomaterial can be improved by incorporating (chemical) crosslinks and/or through the formation of (semi-)interpenetrating polymer networks or double networks. In situ crosslinking can be realized due to f.e. changes in temperature or ion concentration, photo-irradiation or in the presence of enzymes. Materials can also precipitate in situ wherein the polymer has become insoluble due to several physio-chemical changes such as ionic bonding, phase separation, sol-gel transition and as a response to pH. Several injectable systems have already been described in literature to serve soft tissue reconstruction needs (table 2). Several hybrid systems have been described, combining the benefits of the natural materials, namely biological activity, with the tuneability and mechanical strength of synthetic materials.
Table 2. Overview of injectable systems for soft tissue engineering described in literature.
| Crosslinking strategy | Technique | Material | Mechanical properties (kPa) | Swelling ratio (Q) | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Chemical | Click | Oxime click | PEG terminated with amino-oxy functionalities at both sides was combined with HA displaying aldehyde moieties | 0.4–2.9 | 16–20 | Soft and neural tissue engineering | [88] |
| Metal-free triazole linkage | Chitosan and hyaluronan-modified with oxanorbornadiene (OB) and 11-azido-3,6,9-trioxa-undecan-1-amine | 5–41 | 30–40 | Soft tissue engineering | [89] | ||
| Thiol-ene/thiol-yne | Maleimide-functionalized PCL was combined with a thiolated HA | 0.08–0.6 | — | Soft tissue reconstruction | [90] | ||
| Alkyne-functionalized PEG was combined with thiolated alginate | 37 | Load-bearing soft tissue regeneration | [91] | ||||
| Michael addition | Thiolated collagen with an 8-arm PEG-maleimide | 3.9–6.4 | Drug and cell delivery systems | [92] | |||
| Photopolymerisation | N-methacrylate glycol chitosan with a hydrolyzable, hydrophobic, acrylated star-copolymer | 800–1600 | Load-bearing soft tissue regeneration | [93] | |||
| Methacrylated HA combined with green light | 5–146 | Tissue repair | [94] | ||||
| PEG-HA biosynthetic in which the polymerisation is realized via transdermal photo-crosslinking | 11.7 | Soft tissue restoration | [19] | ||||
| Decellularized adipose tissue within photo-crosslinkable methacrylated glycol chitosan or methacrylated chondroitin sulphate | 30–80 | Adipose and soft tissue engineering | [49] | ||||
(Continued)
Table 2. (Continued).
| Crosslinking strategy | Technique | Material | Mechanical properties (kPa) | Swelling ratio (Q) | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Enzyme-mediated | Gelatin-hydroxyphenylpropionic acid/carboxylmethylcellulose-tyramine crosslinked through horseradish peroxidase | 3–4 | 10–40 | Soft tissue engineering | [95] | ||
| Gelatin combined with microbial transglutaminase | 0.01–1.8 | 1.3–4 | Soft tissue regeneration | [96] | |||
| Crosslinking of collagen, chitosan, HA and silica particles which are modified with amine groups via genipin | 0.1–0.3 | 20–40 | Soft tissue engineering | [97] | |||
| Disulphide links | Thiol-functionalized HA and thiol-functionalized human recombinant gelatin cross-linked by poly(ethylene glycol) diacrylate | 0.01–1.7 | — | Neural and soft tissue engineering | [98] | ||
| Poly(N-isopropylacrylamide)-g-chitosan copolymer | 10–58 | 10–16 | Cell-laden injectable system for soft tissue engineering | [99] | |||
| HAuCl4 and a four-armed thiol-terminated PEG | 0.03–4.8 | — | Soft tissue engineering | [100] | |||
| Serum albumin of which the existing disulphide bonds are reduced followed by a re-crosslinking | 0.1–1 | Soft tissue engineering | [101] | ||||
| Ether linkage | HA and human-like collagen, crosslinked via 1,4-butanediol diglycidyl ether | 550 | 47–53 | Soft tissue filler | [102] | ||
| Schiff-base | N-carboxyethyl chitosan combined with oxidized sodium alginate | 0.08–2 | — | Neural soft tissue engineering | [103] | ||
| N,O-carboxymethyl chitosan (O-CMC), combined with aldehyde-functionalized HA | 0.05 | 130 | Abdominal tissue repair | [104] | |||
| CMC and oxidized chondroitin sulphate | 9.5–13 | 20–30 | Drug delivery and tissue engineering | [105] | |||
| Chitosan and PVA with gluteraldehyde as cross‐linker | 18–43 | — | Cell and drug delivery system | [106] | |||
(Continued)
Table 2. (Continued).
| Crosslinking strategy | Technique | Material | Mechanical properties (kPa) | Swelling ratio (Q) | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Redox | Methylcellulose functionalized with methacrylate groups. Polymerisation via ammonium persulfate/ascorbic acid reduction–oxidation reaction | 0.45–1.06 | 33–56 | Soft tissue filler | [107] | ||
| Methacrylated carboxymethylcellulose, crosslinked using ammonium persulfate and ascorbic acid | 0.67–1.78 | 42–72 | Soft tissue filler | [108] | |||
| Free radical cryopolymerisation | Methacrylated HA, methacrylated gelatin, and 4-arm poly(ethylene glycol) acrylate (PEG-4A) in combination with ammonium persulfate as initiator and tetramethylethylenediamine as catalyst | 2–3 | 10–13 | Adipose tissue engineering | [109] | ||
| Physical | Ionic | Alginate crosslinked with a 20 mM Ca2+ solution embedded in fibrin together with cells | 9 | — | Repair and regeneration of soft tissue defects | [110] | |
| Alginate modified with the GRGDY peptide, crosslinked with calcium sulphate | — | Soft tissue engineering | [111] | ||||
| Guanosine 5'‐diphosphate‐crosslinked chitosan | 432–867 | 7–10 | Soft tissue reconstruction and drug delivery | [112] | |||
| Alginate/O-CMC and alginate/PVA | 0.01–1.4 | 2–18 | Adipose tissue engineering | [113] | |||
| Gelatin-alginate crosslinked via ZnSO4 | 50–100 | 10–14 | Drug and cell delivery system | [114] | |||
| Alginate crosslinked by binding to Ca2+ and due to a click reaction between furan-modified alginate and maleimide of a four-arm PEG crosslinker | 0.2–10 | 5–30 | Soft tissue engineering | [115] | |||
| Thermosensitive | Chitosan combined with glycerophosphate salt | — | — | Cosmetics and soft tissue engineering | [116] | ||
| PEG grafted onto a chitosan backbone | — | Drug delivery and soft tissue engineering | [117] | ||||
(Continued)
Table 2. (Continued).
| Crosslinking strategy | Technique | Material | Mechanical properties (kPa) | Swelling ratio (Q) | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Poly(ester urethane urea) | 4–60 | Soft tissue regeneration | [118] | ||||
| Stimuli-responsive | Ionic | B-sheet tape forming peptides combined with chondroitin sulphate | 0.01–80 | Spinal disc regeneration | [119] | ||
| pH | Chitosan and hydroxyapatite composite | 0.2–0.6 | Neovascularisation for tissue regeneration | [120] | |||
| Poly(methyl methacrylate-co-acrylic acid) | 0.3–5.9 | 1–8 | Soft tissue engineering | [121] | |||
| Temperature | PEO90–PPO65–PEO90 multi-block copolymer (F127) | 1.2 | — | Drug delivery and tissue engineering | [122] | ||
| PNVCL | — | 40–140 | Soft tissue engineering | [67] | |||
| Poly(N-isopropylacrylamide)-co-vinylpyrrolidone-co-methacrylate-polylactide | 87–272 | — | Soft tissue repair | [50] | |||
Several parameters are of importance to consider when developing an injectable system suitable for tissue engineering. First and foremost, the mechanical properties are of utmost importance. Indeed, it has been described that cellular fate f.e. of stem cells can be related to mechanical stimuli present in the surrounding micro-environment [123]. Not only do these mechanical cues trigger differentiation, but furthermore they affect cell proliferation as well as cell survival [123, 124]. Therefore, in the process of tissue regeneration, the appropriate mechanical properties when repairing a given tissue are crucial. An overview of the mechanical properties of soft tissues can be seen in table 3.
Table 3. Overview of the mechanical properties of soft tissues.
| Soft tissue | Mechanical properties | Ref |
|---|---|---|
| Muscle | 10–18 kPa | [125, 126] |
| Cartilage | 80–1140 kPa | [127] |
| Brain tissue | 0.1–3 kPa | [125, 128] |
| Skin | 0.2–2 kPa | [129, 130] |
| Adipose tissue | 2–5 kPa | [131–133] |
As can be observed in table 2, the mechanical properties of a material can be tuned based on the starting materials as well as the composition ratio thereof. Several articles have reported on the potential of the developed systems to encapsulate human mesenchymal stem cells (HMSCs), while tuning the mechanical properties of the system. Furthermore, literature has evidenced that mechanical cues can aid to differentiate the stem cells into a specific lineage. For example, Engler et al showed that depending on the matrix in which stem cells were encapsulated, they either exhibited a branched, filopodia-rich morphology, which can be correlated to neural differentiation, compared to a spindle-shaped morphology similar to muscle cells in a 10 times stiffer network [125]. Another essential aspect of tissue regeneration and cell survival is neovascularisation, since encapsulated cells are sensitive to oxygen deprivation. Mechanical properties influence the ability of vasculature to penetrate the construct. Indeed, Li et al showed that with their PCL and HA composite, which has one of the lowest mechanical properties reported, vascular ingrowth can occur more easily when softer systems are applied [90].
Furthermore, not only the mechanical properties of the system are of importance, but also the swelling properties in order to mimic the aqueous environment of cells. The swelling of the material will allow for diffusion of nutrients and waste. However, if the construct swells too much, it could exhibit shape distortion. Most of the injectable systems showed a swelling ratio varying between 10 and 50. Furthermore, a wide spread can be observed for PNVCL since the swelling ratio will be dependent on the temperature at which it was swollen and measured [67]. Moreover, Deng et al described the largest swellable injectable, which can be directly correlated to the low mechanical strength observed [104].
Biocompatibility of the system is of course another important parameter. Most articles described a cell assay either involving seeding or cell encapsulation, showing good cell viability of the crosslinked systems. However, precursor solutions could also have detrimental effects, not shown by these assays. As for click chemistry, all have shifted from the copper-catalysed click chemistry as this will be toxic due to the presence of copper and thus will not find its entry in tissue engineering. Therefore, several other click strategies have been proposed. Fan et al have also selected a metal-free approach consisting of a hybrid system mimicking the ECM of gelatin by using chitosan and hyaluronan mimicking the structure of glycosaminoglycans (GAGs) and the backbone of GAGs respectively [89]. The oxime click reaction resulted in a storage modulus of around 1–3 kPa, in line with the nervous system, more specifically brain tissue [88]. By incorporation of collagen type I in the system, cell-interactivity could be achieved. Furthermore, some of the crosslinking techniques described pose several disadvantages for (soft) tissue engineering. Photo-polymerisation requires a light-emitting source to initiate the polymerisation which can only be realized via open surgeries, although a new technique has already been described in which transcutaneous emission has been offered as an alternative [19]. Most of the photo-initiators used, have an excitation maximum between 270 and 405 nm [134]. Caution needs to be taken when exposing viable, healthy tissue to UV-A light, as cells might be affected which causes problems later on as reflected f.e. by cellular mutations [135]. Lastly, since most of the crosslinking occurs in situ, it is evident that reactive oxygen species which are known to cause cellular changes on a DNA level, could potentially result in detrimental effects in the long term [136]. There are however several mitigation strategies described to minimize the risks of cytotoxic effects. On the one hand, the hydrogel or polymer itself can be optimized based on hydrophilicity, electrical charge, shape and molar mass. Moreover, as a rough surface is more prone to infection and inflammation, roughness and topography of materials can be tuned. The chemistry and polymerisation can also influence inflammation and can thus be optimized [137–139].
The gelation time also needs to be taken into account, since migration of the precursors can potentially occur. Tissue adhesion, by incorporation of cell-interactive sequences, such as RGD, could be a tool to avoid or minimize migration of the injectable. Here, the RGD sequences will bind to integrins present on the cell membrane, anchoring the material. Another option to avoid migration and overcome slow gelation times is the use of 'pre-formed' injectable systems that have shear thinning properties [101]. Due to shear stress, the viscosity of the material will decrease, making the system suitable after injection. Upon relief of the shear stress, the material will self-heal and return to its original structure. However, the mechanical properties of these shear thinning constructs are often relatively low <1 kPa [101, 140]. This can be attributed to the fragility of the physically crosslinked system [141].
The degradation is generally also closely associated with the mechanical properties of a material and this aspect plays a crucial role in the tissue engineering process. If a material breaks down too fast, no cellular infiltration can occur, while on the other hand, chronic inflammation is sometimes observed for permanent, non-resorbable implants. As can be anticipated, degradation will generally occur slower for stiffer biomaterials since more crosslinks are incorporated within the system resulting in a denser network and thus longer degradation times. Enzyme-based crosslinking approaches have resulted in mechanically weaker gels and were accompanied by fast disintegration and/or a mechanical strength decrease of the applied scaffolds during in vitro culture of adipose tissue-derived stem cells [96]. Moreover, a short shelf life has already been observed for most peptides as they are instable, especially at room temperature [142]. Furthermore, several of the physically crosslinked networks will result in mechanically weak gels which can therefore not be used for any load-bearing applications. However, these systems are known to exert excellent biocompatibility and can withstand body temperature and pH changes [143]. Biomaterials that are prepared via a Michael-type addition reaction mostly suffer from long gelation times, rendering them prone towards flow/spreading of cells and material outside of the targeted region [144].
The injectability of most of these systems can be found in the use of precursor systems that will form a gel in situ. Hayami et al focussed on the applied force needed for injection through different needle sizes. Depending on the needle size used, it was observed that a larger needle size (16 G) will result in a smaller injection force needed, namely 14 N, compared to 55 N for a 20 G needle [93]. Other articles did report on the viscosity. For example, Fenn et al reported on viscosity in function of shear stress of both 100 kDa and 700 kDa HA-MA, varying between 0.01 and 10 Pa s dependant on both molar mass and degree of substitution [94]. Several other articles obtained viscosities between, 0.35 and 149.2 Pa s; 0.982 Pa s and 0.03–2.2 Pa s for crosslinked methylcellulose hydrogels [107], a carboxymethylcellulose hydrogel [108] and a thermosensitive PEG grafted chitosan respectively. Lastly, Kocak et al performed an extensive (although subjective) injectability assay wherein different compositions and different needle sizes were assessed on good flow to no flow. One of the shortcomings of most articles is the fact that although stating that an injectable system is developed, viscosity measurements, injection force or proof of injectability are lacking.
6. Conclusion
A wide variety of injectable systems has been described in literature for numerous soft tissue applications. Several advances have been accomplished showing promise towards injectable ECM mimics able to support cells. However, concerns remain towards clinical translation of the injectables. The limited longevity, scalability and inflammation can be detrimental towards clinical translation. Natural polymers have been used for cell and drug delivery as well as temporary fillers, however, a lack of mechanical integrity has been reported as well as a speedy degradation profile. Tailorable mechanical properties and degradation times could be obtained with synthetic polymers. Furthermore, a plethora of crosslinking strategies have been used over the years, trying to improve the stability of the injectables as well as their mechanical properties. It can be concluded that although several drawbacks have been solved, there are still limitations towards successful long-term clinical translation. Composite/hybrid systems entailing both natural and synthetic polymers could offer a promising alternative as both bioactivity and the desired mechanical properties can be achieved.