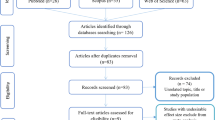
Abstract
The relationship of intrauterine phthalate exposure with gestational metabolic syndrome (GMS) parameters is inconsistently reported. We performed a systematic review to evaluate the association between prenatal phthalate exposure and GMS parameters. A literature search was performed in three databases. According to the defined PECOS statement, eligible studies were identified. The method and result for each study was qualitatively summarized with great emphasis on study design and exposure assessment. Fourteen studies were included in the present systematic review. Two studies used one-spot serum sample for evaluation of phthalate exposure, while others used 1–4 urine samples. Concentrations of phthalate metabolites varied substantially, and the levels in serum were greatly lower than those in urine. These studies observed no interstudy or intrastudy consistency for association between phthalates and GMS in pregnant women cross-sectionally or longitudinally, regardless of phthalates species or GMS indicator. Most reported associations were not significantly different from null result. Besides, positive and negative relationships also existed. The current epidemiological data do not support the hypothesis that prenatal exposure to phthalates increases GMS risk.

Similar content being viewed by others
References
Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R (2008) Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect 116(4):467–473
Alves A, Kucharska A, Erratico C, Xu F, Den Hond E, Koppen G, Vanermen G, Covaci A, Voorspoels S (2014) Human biomonitoring of emerging pollutants through non-invasive matrices: state of the art and future potential. Anal Bioanal Chem 406(17):4063–4088
Alves A, Covaci A, Voorspoels S (2016) Are nails a valuable non-invasive alternative for estimating human exposure to phthalate esters? Environ Res 151:184–194
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL (2005) Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200
Ben-Haroush A, Yogev Y, Hod M (2004) Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med 21(2):103–113
Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota DTL, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY, Alshawabkeh AN, Meeker JD (2014) Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int 62:1–11
Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF (2016) Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ Health Perspect 10(124):1651–1655
Chang YJ, Lin KL, Chang YZ (2013) Determination of Di-(2-ethylhexyl)phthalate (DEHP) metabolites in human hair using liquid chromatography-tandem mass spectrometry. Clin Chim Acta 420:155–159
Dukler D, Porath A, Bashiri A, Erez O, Mazor M (2001) Remote prognosis of primiparous women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 96(1):69–74
Ehrenthal DB, Maiden K, Rogers S, Ball A (2014) Postpartum healthcare after gestational diabetes and hypertension. J Women's Health (Larchmt) 23(9):760–764
Fisher BG, Frederiksen H, Andersson A, Juul A, Thankamony A, Ong KK, Dunger DB, Hughes IA, Acerini CL (2018) Serum phthalate and triclosan levels have opposing associations with risk factors for gestational diabetes mellitus. Front Endocrinol 9:99
Frederiksen H, Skakkebaek NE, Andersson AM (2007) Metabolism of phthalates in humans. Mol Nutr Food Res 51(7):899–911
Gao H, Wu W, Xu Y, Jin Z, Bao H, Zhu P, Su P, Sheng J, Hao J, Tao F (2017a) Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Sci Rep 7(1):13106
Gao H, Xu YY, Huang K, Ge X, Zhang YW, Yao HY, Xu YQ, Yan SQ, Jin ZX, Sheng J, Zhu P, Hao JH, Tao FB (2017b) Cumulative risk assessment of phthalates associated with birth outcomes in pregnant Chinese women: A prospective cohort study. Environ Pollut 222:549–556
Gao H, Zhang YW, Huang K, Yan SQ, Mao LJ, Ge X, Xu YQ, Xu YY, Sheng J, Jin ZX, Zhu P, Tao XG, Hao JH, Tao FB (2017c) Urinary concentrations of phthalate metabolites in early pregnancy associated with clinical pregnancy loss in Chinese women. Sci Rep 7(1):6800
Gao H, Zhu YD, Xu YY, Zhang YW, Yao HY, Sheng J, Jin ZX, Ren LL, Huang K, Hao JH, Tao FB (2017d) Season-dependent concentrations of urinary phthalate metabolites among Chinese pregnant women: Repeated measures analysis. Environ Int 104:110–117
Golestanzadeh M, Riahi R, Kelishadi R (2019) Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environ Sci Pollut Res Int 26(35):35670–35686
Greenfield DM, Snowden JA (2019) Cardiovascular diseases and metabolic syndrome. In Carreras E, Dufour C, Mohty M, Kröger N (Eds.), The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies [Internet]. Cham: Springer pp 415-420
Grun F, Blumberg B (2009) Endocrine disrupters as obesogens. Mol Cell Endocrinol 304(1-2):19–29
Gupta A, Gupta V (2010) Metabolic syndrome: what are the risks for humans? Biosci Trends 4(5):204–212
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924
Han X, Li J, Wang Y, Xu S, Li Y, Liu H, Zhou Y, Zhao H, Fang J, Cai Z, Xia W (2020) Association between phthalate exposure and blood pressure during pregnancy. Ecotox Environ Safe 189:109944
Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM (2004) Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 112(17):1734–1740
Heindel JJ, Vom SF, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, Legler J, La Merrill M, Rizzir L, Machtinger R, Mantovani A, Mendez MA, Montanini L, Molteni L, Nagel SC, Parmigiani S, Panzica G, Paterlini S, Pomatto V, Ruzzin J, Sartor G, Schug TT, Street ME, Suvorov A, Volpi R, Zoeller RT, Palanza P (2015) Parma consensus statement on metabolic disruptors. Environ Health 14:54
Hsu JY, Ho HH, Liao PC (2015) The potential use of diisononyl phthalate metabolites hair as biomarkers to assess long-term exposure demonstrated by a rat model. Chemosphere 118:219–228
James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, McElrath TF, Seely EW (2016) Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int 96:118–126
James-Todd TM, Chiu Y, Messerlian C, Mínguez-Alarcón L, Ford JB, Keller M, Petrozza J, Williams PL, Ye X, Calafat AM, Hauser R (2018) Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health-Glob 17(1):55
Jensen MS, Anand-Ivell R, Norgaard-Pedersen B, Jonsson BA, Bonde JP, Hougaard DM, Cohen A, Lindh CH, Ivell R, Toft G (2015) Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 26(1):91–99
Kim SH, Park MJ (2014) Phthalate exposure and childhood obesity. Ann Pediatr Endocrinol Metab 19(2):69–75
Kim J, Cha S, Lee MY, Hwang YJ, Yang E, Ryou C, Jung HI, Cheon YP (2018) Chronic Low-Dose Nonylphenol or Di-(2-ethylhexyl) Phthalate has a different estrogen-like response in mouse uterus. Dev Reprod 22(4):379–391
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Lohsiriwat S, Imrittha N (2008) Effect of posture on creatinine clearance in late pregnancy and after pregnancy. J Obstet Gynaecol Res 34(3):337–342
MacPherson S, Arbuckle TE, Fisher M (2018) Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 28(5):481–493
Martin J, Moder M, Gaudl A, Alonso E, Reemtsma T (2015) Multi-class method for biomonitoring of hair samples using gas chromatography-mass spectrometry. Anal Bioanal Chem 407(29):8725–8734
Mose T, Mortensen GK, Hedegaard M, Knudsen LE (2007) Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod Toxicol 23(1):83–91
Pan G, Hanaoka T, Yu L, Na J, Yamano Y, Hara K, Ichiba M, Nakadate T, Kishi R, Wang P, Yin H, Zhang S, Feng Y (2011) Associations between hazard indices of di-n-butylphthalate and di-2-ethylhexylphthalate exposure and serum reproductive hormone levels among occupationally exposed and unexposed Chinese men. Int J Androl 34(5 Pt 2):e397–e406
Park C, Lee J, Kong B, Park J, Song H, Choi K, Guon T, Lee Y (2019) The effects of bisphenol A, benzyl butyl phthalate, and di(2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Environ Pollut 248:774–781
Philips EM, Trasande L, Kahn LG, Gaillard R, Steegers EAP, Jaddoe VWV (2019) Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum Reprod 34(2):365–373
Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L, Jaddoe VWV (2020) Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environ Int 135:105342
Radke EG, Braun JM, Nachman RM, Cooper GS (2020) Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ Int 137:105408
Robledo CA, Peck JD, Stoner J, Calafat AM, Carabin H, Cowan L, Goodman JR (2015) Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int J Hyg Environ Health 218(3):324–330
Sattar N, Greer IA (2002) Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 325(7356):157–160
Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, Swan SH, Barrett ES, Nguyen R, Bush N, McElrath TF, Sathyanarayana S (2019) Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int 123:588–596
Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, Taback S, Keely E, Bouchard MF, Monnier P, Dallaire R, Morisset A, Ettinger AS (2015) Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int 83:63–71
Sheng Y (2019) Phthalate levels in pregnant women serum and risk of hypertensive disorder complicating pregnancy. Guangxi: Guangxi Medical University (Master thesis in Chinese)
Silva MJ, Reidy JA, Herbert AR, Preau JJ, Needham LL, Calafat AM (2004) Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol 72(6):1226–1231
Tefre DRK, Frederiksen H, Christensen JS, Boye KH, Andersson AM, Husby S, Barington T, Main KM, Jensen TK (2014) Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction 147(4):443–453
Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M (2015) Prenatal phthalate exposure and childhood growth and blood pressure: evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect 123(10):1022–1029
Villanger GD, Drover S, Nethery RC, Thomsen C, Sakhi AK, Overgaard KR, Zeiner P, Hoppin JA, Reichborn-Kjennerud T, Aase H, Engel SM (2020) Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ Int 137:105509
Wang JQ, Gao H, Sheng J, Tao XY, Huang K, Zhang YW, Mao LJ, Zhou SS, Jin ZX, Tao FB (2020a) Urinary concentrations of phthalate metabolites during gestation and intrahepatic cholestasis of pregnancy: a population-based birth cohort study. Environ Sci Pollut Res Int 27(11):11714–11723
Wang JQ, Hu YB, Gao H, Sheng J, Huang K, Zhang YW, Mao LJ, Zhou SS, Cai XX, Zhang LJ, Wang SF, Hao JH, Yang LQ, Tao FB (2020b) Sex-specific difference in placental inflammatory transcriptional biomarkers of maternal phthalate exposure: a prospective cohort study. J Expo Sci Environ Epidemiol 30(5):835–844
Warembourg C, Basagaña X, Seminati C, de Bont J, Granum B, Lyon-Caen S, Manzano-Salgado CB, Pin I, Sakhi AK, Siroux V, Slama R, Urquiza J, Vrijheid M, Thomsen C, Casas M (2019) Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int J Hyg Environ Health 222(3):446–454
Wen SW, Xie RH, Tan H, Walker MC, Smith GN, Retnakaran R (2012) Preeclampsia and gestational diabetes mellitus: pre-conception origins? Med Hypotheses 79(1):120–125
Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP (2015) The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: the HOME Study. Environ Health-Glob 14:75
Xiong X, Saunders LD, Wang FL, Demianczuk NN (2001) Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet 75(3):221–228
Yamaoka K, Tango T (2012) Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med 10:138
Yao HY, Han Y, Gao H, Huang K, Ge X, Xu YY, Xu YQ, Jin ZX, Sheng J, Yan SQ, Zhu P, Hao JH, Tao FB (2016) Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 157:42–48
Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, Niu Y, Du L (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8(1):2–10
Zhang Y, Gao H, Huang K, Xu Y, Sheng J, Tao F (2017) A cohort study on association between the first trimester phthalates exposure and fasting blood glucose level in the third trimester. Chin J Epidemiol (Chinese) 3(38):388–392
Zhao R, Wu Y, Zhao F, Lv Y, Huang D, Wei J, Ruan C, Huang M, Deng J, Huang D, Qiu X (2017) The risk of missed abortion associated with the levels of tobacco, heavy metals and phthalate in hair of pregnant woman: a case control study in Chinese women. Medicine (Baltimore) 96(51):e9388
Zhu YD, Gao H, Huang K, Zhang YW, Cai XX, Yao HY, Mao LJ, Ge X, Zhou SS, Xu YY, Jin ZX, Sheng J, Yan SQ, Pan WJ, Hao JH, Zhu P, Tao FB (2018) Prenatal phthalate exposure and placental size and shape at birth: a birth cohort study. Environ Res 160:239–246
Funding
This work is supported by 2020 Cultivation Project for Youth Science Fund from the First Affiliated Hospital of Anhui Medical University (No. 2020kj02); Clinical Scientific Research Project of Anhui Medical University (No. 2020xkj161).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
GH searched the database, identified eligible studies and wrote the manuscript. ZC extracted the main statistics, and wrote the manuscript. TFB wrote and checked the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Gao, H., Zhang, C. & Tao, FB. Association between prenatal phthalate exposure and gestational metabolic syndrome parameters: a systematic review of epidemiological studies. Environ Sci Pollut Res 28, 20921–20938 (2021). https://doi.org/10.1007/s11356-021-13120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13120-4