Abstract
In the present study, the nearest-neighbor tight-binding model has been employed to calculate the density of states (DOS), electronic heat capacity (EHC), and Pauli magnetic susceptibility (PMS) of hydrogenated systems, namely monolayer graphone and graphane, bilayer graphone–graphene, and bilayer graphane–graphene. Then, the results have been compared with that of monolayer and simple bilayer graphene. It was found that the behaviors of hydrogenated systems differ from those of monolayer and bilayer graphene near the Fermi Level. Also, monolayer graphone and bilayer graphone–graphene exhibit a high peak near the Fermi level. Graphane monolayer, on the other hand, has no states around the Fermi level. Furthermore, bilayer graphane–graphone, similar to graphene, is a semimetal. Also, Schottky anomaly peaks in the EHC curves and crossovers in the PMS curves can be observed, which have divided the domain into two regions of low and high temperature. Compared to hydrogenated systems, the Schottky anomaly in graphene monolayer and bilayer graphene occurred at lower temperatures, while the PMS of monolayer graphone and bilayer graphone–graphene were faster than other systems in reaching the crossover. From the theoretical standpoint, these phenomena are due to the proportional relation of the PMS and EHC with the DOS.




Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA (2005) Two-dimensional gas of massless Dirac fermions in graphene. Nature 438:197–200
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Castro Neto AH, Guinea F, Peres NMR, Novoselov KS, Geim AK (2009) The electronic properties of graphene. Rev Mod Phys 81:109
Soldano C, Mahmood A, Dujardin E (2010) Production, properties and potential of graphene. Carbon 48:2127–2150
Angel J, Guillen S, Guinea F (2020) Electron heating and mechanical properties of graphene. Phys Rev B 101:060102
Boukhvalov DW, Katsnelson MI (2009) Chemical functionalization of graphene. J Phys Condens Matter 21:344205
Sevincli H, Sevik C, Cagin T, Cuniberti G (2013) A bottom-up route to enhance thermoelectric figures of merit in graphene nanoribbons. Sci Rep 3:1–6
Wakabayashi K, Sasaki KI, Nakanishi T, Enoki T (2010) Electronic states of graphene nanoribbons and analytical solutions. Sci Technol Adv Mater 11:054504
Elias DC, Nair RR, Mohiuddin TMG, Morozov SV, Blake P, Halsall MP, Ferrari AC, Boukhvalov DW, Katsnelson MI, Geim AK, Novoselov KS (2009) Control of graphene’s properties by reversible hydrogenation: evidence for graphane. Science 323:610–613
Shih PH, Do TN, Gumbs G, Lin MF (2020) Electronic and optical properties of doped graphene. Phys E 118:113894
Mousavi H, Khodadadi J, Grabowski M (2017) Semiconducting behavior of substitutionally doped bilayer graphene. Phys B 530:90–94
Zhou J, Wang Q, Sun Q, Chen XS, Kawazoe Y, Jena P (2009) Ferromagnetism in semihydrogenated graphene sheet. Nano Lett 9:3867–3870
Wu M, Burton JD, Tsymbal EY, Zeng XC, Jena P (2013) Hydroxyl-decorated graphene systems as candidates for organic metal-free ferroelectrics, multiferroics, and high-performance proton battery cathode materials. Phys Rev B 87:081406
Sofo JO, Chaudhari AS, Barber GD (2007) Graphane: a two-dimensional hydrocarbon. Phys Rev B 75:153401
Zhou J, Sun Q (2012) How to fabricate a semihydrogenated graphene sheet? A promising strategy explored. Appl Phys Lett 101:073114
Costamagna S, Neek-Amal M, Los JH, Peeters FM (2012) Thermal rippling behavior of graphane. Phys Rev B 86:041408
Leenaerts O, Partoens B, Peeters FM (2009) Hydrogenation of bilayer graphene and the formation of bilayer graphane from first principles. Phys Rev B 80:245422
Schwierz F (2010) Graphene transistors. Nat Nanotech 5:487
Brumfiel G (2009) Graphene gets ready for the big time. Nature 458:390–391
Balog R, Jorgensen B, Nilsson L, Andersen M, Rienks E, Bianchi M, Fanetti M, Lagsgaard E, Baraldi A, Lizzit S, Sljivancanin Z (2010) Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat mater 9:315–319
Haberer DVVD, Vyalikh DV, Taioli S, Dora B, Farjam M, Fink J, Marchenko D, Pichler T, Ziegler K, Simonucci S, Dresselhaus MS (2010) Tunable band gap in hydrogenated quasi-free-standing graphene. Nano Lett 10:3360–3366
Kharche N, Nayak SK (2011) Quasiparticle band gap engineering of graphene and graphone on hexagonal boron nitride substrate. Nano Lett 11:5274–5278
Xie L, Wang X, Lu J, Ni Z, Luo Z, Mao H, Wang R, Wang Y, Huang H, Qi D, Liu R, Yu T, Shen Z, Wu T, Peng H, Özyilmaz B, Loh K, Wee ATS, Ariando Chen W (2011) Room temperature ferromagnetism in partially hydrogenated epitaxial graphene. Appl Phys Lett 98:1931139
Feng L, Zhang WX (2012) The structure and magnetism of graphone. AIP Adv 2:042138
Boukhvalov DW (2010) Stable antiferromagnetic graphone. Phys E 43:199–201
Gmitra M, Kochan D, Fabian J (2013) Spin-orbit coupling in hydrogenated graphene. Phys Rev Lett 110:246602
Pujari BS, Gusarov S, Brett M, Kovalenko A (2011) Single-side-hydrogenated graphene: Density functional theory predictions. Phys Rev B 84:041402
Podlivaev AI, Openov LA (2011) On the thermal stability of graphone. Semiconductors 45:958–961
Kaxiras E (2003) Atomic and electronic structure of solids. Cambridge University Press, Cambridge
Ashcroft WN, Mermin ND (1976) Solid state physics. Harcout college Publishers, New York
Grosso G, Parravicini GP (2000) Solid state physics. Academic Press, New York
Mousavi H, Jalilvand S, Kurdestany JM, Grabowski M (2017) Electron doping effects on the electrical conductivity of zigzag carbon nanotubes and corresponding unzipped armchair graphene nanoribbons. Phys E 94:87–91
Mousavi H, Khodadadi J (2015) Graphene to graphane: two-band approach. Superlattice Microst 88:434–441
Kittel C (2004) Introduction to solid state physics, 8th edn. Wiley, New York
Mousavi H, Khodadadi J, Grabowski M (2015) Electronic properties of long DNA nanowires in dry and wet conditions. Solid State Commun 222:42–48
Nolting W, Ramakanth A (2009) Quantum theory of magnetism. Springer, New York
Mousavi H, Jalilvand S (2019) Electrical and thermal conductivities of few-layer armchair graphene nanoribbons. Euro Phys J B 92:1–11
Mousavi H, Jalilvand S, Mirzaei F (2019) Magnetic and thermal characteristics of armchair graphene nanoribbons in the two-band Harrison model. J Magn Magn Mater 469:405–410
Sohrabi Sani S, Mousavi H, Asshabi M, Jalilvand S (2020) Electronic properties of graphyne and graphdiyne in tight-binding model. ECS J Solid State Sci Tech 9:031003
Mousavi H, Khodadadi J, Kurdestany JM, Yarmohammadi Z (2016) Electrical and thermal conductivities of the graphene, boron nitride and silicon boron honeycomb monolayers. Phys Lett A 380:3823–3827
Mousavi H, Jalilvand S, Kurdestany JM (2016) Pauli magnetic susceptibility of bilayer graphene and hexagonal boron-nitride. Phys B 502:132–139
Jalilvand S, Mousavi H (2020) Multi-band tight-binding model of \({\rm MoS}_{2}\) monolayer. J Elec Mater 49:3599–3608
Bruus H, Flensberg K (2004) Many-body quantum theory in condensed matter physics: an introduction. Oxford University Press, Oxford
Karlicky F, Zboril R, Otyepka M (2012) Band gaps and structural properties of graphene halides and their derivates: a hybrid functional study with localized orbital basis sets. J Chem Phys 137:034709
Karlicky F, Otyepka M (2013) Band gaps and optical spectra of chlorographene, fluorographene and graphane from \({\rm G}_{0}{\rm W}_{0}\), \({\rm GW}_{0}\) and GW calculations on top of PBE and HSE06 orbitals. J Chem Theory Comput 9:4155–4164
Hu JQ, Xu LH, Wu SQ, Zhu ZZ (2019) Electronic and optical properties of graphane, silicane, MoS2 homo-bilayers and hetero-bilayers. Curr Appl Phys 19:1222–1232
Son J, Lee S, Kim SJ, Park BC, Lee HK, Kim S, Kim JH, Hong BH, Hong J (2016) Hydrogenated monolayer graphene with reversible and tunable wide band gap and its field-effect transistor. Nat Commun 7:1–7
Schleder GR, Marinho E Jr, Baquiao DJ, Celaschi YM, Gollino F, Dalpian GM, Autreto PA (2020) Tuning hydrogen adsorption and electronic properties from graphene to fluorographone. Phys Rev Mater 4:074005
Li S, Li J, Wang Y, Yu C, Li Y, Duan W, Wang Y, Zhang J (2020) Electric-Field Control of Giant On/Off Current Ratio in Graphene Through Reversible Hydrogenation. arXiv preprint arXiv:2008.09749
Li H, Papadakis R, Hussain T, Karton A, Liu J (2020) Moiré patterns arising from bilayer graphone/graphene superlattice. Nano Res 13:1060–1064
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Graphene
The lattice structure of graphene monolayer is shown in Fig. 5a. As observed, the BLUC of graphene includes two nonequivalent atoms. Therefore, the TB Hamiltonian of this structure is represented by a \(2\times 2\) matrix:
1.2 Graphane
The lattice structure of graphane monolayer is illustrated in Fig. 6a. According to this figure, the BLUC of graphane contains four nonequivalent atoms. Therefore, the TB Hamiltonian of this structure is given by a \(4\times 4\) matrix:
1.3 Bilayer graphene
According to Fig. 5b, the BLUC of the simple bilayer graphene (AA-stacked) includes four nonequivalent atoms. Therefore, the TB Hamiltonian of the bilayer graphene would be shown by a \(4\times 4\) matrix:
in which \(t_\bot =t_0/7\) is the inter-layer hopping parameter between the carbon atoms [32].
1.4 Bilayer graphone–graphene
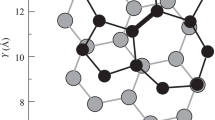
As it is shown in Fig. 1b, the BLUC of bilayer graphone–graphene (AA-stacked) includes five nonequivalent atoms. Therefore, the TB Hamiltonian of this structure is represented by a \(5\times 5\) matrix:
where \(t_\bot =t_0/7\) is inter-layer hopping parameter between pristine carbon atoms [32], and \(t^{\prime }_\bot =3t_0/14\) refers to the inter-layer hopping parameter between the carbon atoms in graphene layer and the carbon atoms bonded to the hydrogen atoms in the graphone layer.
1.5 Bilayer graphane–graphene
According to Fig. 6b, since the BLUC of bilayer graphane–graphene (AA-stacked) includes six nonequivalent atoms, its TB Hamiltonian is given by a \(6\times 6\) matrix:
Rights and permissions
About this article
Cite this article
Sohrabi Sani, S., Mousavi, H., Jalilvand, S. et al. Hydrogenation effects on the thermal and magnetic properties of mono- and bilayer graphene. Carbon Lett. 31, 1089–1096 (2021). https://doi.org/10.1007/s42823-021-00227-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-021-00227-4