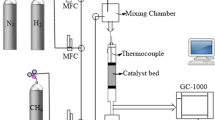
Abstract
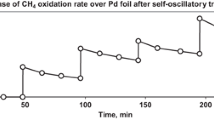
Self-oscillatory methane oxidation over Ni foil at 750°C for 1 h resulted in the formation of a porous layer with a depth of 10–12 μm. The layer depth did not increase as the self-oscillatory reaction time was increased to 2–3 h. The porous layer consisted of nickel oxide crystals 100–200 nm in size or metallic Ni crystallites (indistinct crystals) of the above size in an oxidative gas atmosphere or a reducing atmosphere, respectively. The formation of the layer caused a great increase in the catalytic activity of nickel in the carbon dioxide conversion of methane (CDCM). Immediately after the self-oscillatory reaction, the crystals in the porous layer were in an oxidized state. In this state, they can remain at 750°C in a flow of an inert gas or CO2 without catalytic activity losses. On the contrary, the oxide crystals were reduced to the metal in a reducing atmosphere (H2, CH4), and they gradually stuck together to form a spongy structure whose surface area was much lower than that of the initial oxidized sample. A decrease in the catalytic activity of nickel after reducing pretreatment and in the course of the catalytic CDCM (where the catalyst was also reduced) confirmed the above conclusion. The porous layer on the Ni surface was similar to a Ni foam sample with pores of ~1 μm (metallic membrane) in catalytic and reactive properties, but it differed in a limited depth on the surface of the bulk metal.






Similar content being viewed by others
REFERENCES
Ashok, J., Wai, M.H., and Kawi, S., ChemCatChem, 2018, vol. 10, p. 3927.
Bkour, Q., Marin-Flores, O.G., Graham, T.R., Ziaei, P., Saunders, S.R., Norton, M.G., and Ha, S., Appl. Catal., A, 2017, vol. 546, p. 126.
Li, J. and Lu, G., Appl. Catal., A, 2004, vol. 273, p. 163.
Yao, Y.-F.Y. and Kummer, J.T., J. Catal., 1973, vol. 28, p. 124.
Seo, H.O., Catalysts, 2018, vol. 8, p. 110.
Bychkov, V.Yu., Krylov, O.V., and Korchak, V.N., Kinet. Catal., 2002, vol. 43, no. 1, p. 86.
Simonov, M.N., Rogov, V.A., Smirnova, M.Yu., and Sadykov, V.A., Catalysts, 2017, vol. 7, p. 268.
Matus, E.V., Sukhova, O.B., Ismagilov, I.Z., Ushakov, V.A., Yashnik, S.A., Kerzhentsev, M.A., Ismagilov, Z.R., Nefedova, D.V., and Nikitin, A.P., Kinet. Catal., 2019, vol. 60, no. 4, p. 496.
Matus, E.V., Shlyakhtina, A.S., Sukhova, O.B., Ismagilov, I.Z., Ushakov, V.A., Yashnik, S.A., Kerzhentsev, M.A., Ismagilov, Z.R., Nikitin, A.P., and Bharali, P., Kinet. Catal., 2019, vol. 60, no. 2, p. 221.
Jalama, K., Catal. Rev., 2017, vol. 59, no. 2, p. 95.
Popov, Y.V., Mokhov, V.M., Nebykov, D.N., Latyshova, S.E., Shcherbakova, K.V., and Panov, A.O., Kinet. Catal., 2018, vol. 59, no. 4, p. 444.
Chesnokov, V.V., Chichkan, A.S., Paukshtis, E.A., Chesalov, Y.A., and Krasnov, A.V., Kinet. Catal., 2019, vol. 60, no. 4, p. 439.
Golubina, E.V., Lokteva, E.S., Kavalerskaya, N.E., and Maslakov, K.I., Kinet. Catal., 2020, vol. 61, no. 3, p. 444.
Zhang, X.L., Hayward, D.O., and Mingos, D.M.P., Catal. Lett., 2002, vol. 83, p. 149.
Tulenin, Yu.P., Sinev, M.Yu., Savkin, V.V., and Korchak, V.N., Catal. Today, 2004, vols. 91–92, p. 155.
Gladky, A.Yu., Kaichev, V.V., Ermolaev, V.K., Bukhtiyarov, V.I., and Parmon, V.N., Kinet. Catal., 2005, vol. 46, p. 251.
Bychkov, V.Yu., Tyulenin, Yu.P., Korchak, V.N., and Aptekar, E.L., App. Catal., A, 2006, vol. 30, p. 21.
Saraev, A.A., Vinokurov, Z.S., Shmakov, A.N., Kaichev, V.V., and Bukhtiyarov, V.I., Kinet. Catal., 2018, vol. 59, no. 6, p. 810.
Bychkov, V.Yu., Tulenin, Yu.P., Slinko, M.M., Gordienko, Yu.A., and Korchak, V.N., Catal. Lett., 2018, vol. 148, p. 653.
Kaichev, V.V., Teschner, D., Saraev, A.A., Kosolobov, S.S., Gladky, A.Yu., Prosvirin, I.P., Rudina, N.A., Ayupov, A.B., Blume, R., Hövecker, M., Knop-Gericke, A., Schlögl, R., Latyshev, A.V., and Bukhtiyarov, V.I., J. Catal., 2016, vol. 334, p. 23.
Saraev, A.A., Kaichev, V.V., Bukhtiyarov, V.I., and Kosolobov, S.S., Kinet. Catal., 2015, vol. 56, no. 5, p. 598.
Kaichev, V.V., Gladky, A.Yu., Saraev, A.A., Kosolobov, S.S., Sherstyuk, O.V., and Bukhtiyarov, V.I., Top. Catal., 2020, vol. 63, p. 24.
Bychkov, V.Yu., Tulenin, Yu.P., Slinko, M.M., Sokolov, S., and Korchak, V.N., Catal. Lett., 2017, vol. 147, p. 1019.
Bychkov, V.Yu., Tyulenin, Yu.P., Slinko, M.M., and Korchak, V.N., Appl. Catal., A, 2007, vol. 321, p. 180.
Saraev, A.A., Vinokurov, Z.S., Kaichev, V.V., Shmakov, A.N., and Bukhtiyarov, V.I., Catal. Sci. Technol., 2017, vol. 7, p. 1646.
Funding
This work was supported by the Russian Foundation for Basic Research (grant no. 19-03-00096) and carried out within the framework of state contract V. 46.13, 0082-2014-007 (project no. AAAA-A18-11802089010503).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Abbreviations: CDCM, carbon dioxide conversion of methane; SEM, scanning electron microscopy.
Rights and permissions
About this article
Cite this article
Bychkov, V.Y., Tulenin, Y.P., Gorenberg, A.Y. et al. Investigation of Nickel Surface Layers Formed in the Course of Self-Oscillatory Methane Oxidation. Kinet Catal 62, 181–187 (2021). https://doi.org/10.1134/S0023158421010018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158421010018