Abstract
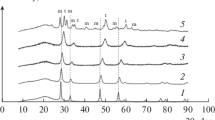
The 5% CuO/Ce1 – xPrxOy catalysts were synthesized on the basis of CeO2 and PrO2 oxides and Ce1 – xPrxOy solid solutions with x = 0.2, 0.5, and 0.8. Highly dispersed copper oxide was present in the 5%CuO/Ce1 – xPrxOy catalysts. Upon interaction with the support, it formed active oxygen, which participated in CO chemisorption and a low-temperature reaction of CO oxidation in the presence of hydrogen. The highest conversion of CO in an excess of H2 (γmах(Т)), which was close to 100%, was obtained at temperatures of 120–160°C on a 5% CuO/CeO2 catalyst. Upon the modification of CeO2 with Pr cations, 5% Ce0.2Pr0.8Oy sample, it decreased to 65% at 220°C due to an increase in the bond strength of oxygen in copper-containing centers. A maximum conversion of CO (93%) on a sample of 5% CuO/PrOy was detected at 200°C. Upon the modification of PrO2 with Ce cations, the activity of 5% CuO/Ce0.5Pr0.5Oy and 5% CuO/Ce0.2Pr0.8Oy catalysts did not exceed that of 5% CuO/PrOy. The forms of CO and CO2 adsorption on 5% CuO/Ce1 – xPrxOy samples were studied using the TPD method. In a range of 170–500°C, the desorption of oxygen from the supports of 5% CuO/Ce0.5Pr0.5Oy and 5% CuO/PrOy samples was observed. The occurrence of the reaction on 5% CuO/Ce1 – xPrxOy catalysts was discussed. With consideration for the properties of CO complexes formed on copper-containing oxidation and adsorption centers, their participation in the reaction of low-temperature oxidation in hydrogen was examined.








Similar content being viewed by others
REFERENCES
Olah, G.A., Goeppert, A., and Prakash, S., Beyond Oil and Gas: The Methanol Economy, Weinheim: Wiley, 2006.
Mishra, A. and Prasad, R., Bull. Chem. React. Eng. Catal., 2011, vol. 6, no. 1, p. 1.
Yu, K., Lou, L.-L., Liu, S., and Zhou, W., Adv. Sci., 2020, vol. 7, p. 1.
Martinez-Arias, A., Gamarra, D., Hungria, A.B., Fernandez-Garcia, M., Munuera, G., Hornes, A., Bera, P., Conesa, J.C., and Camara, A.L., Catalysts, 2013, vol. 3, p. 378.
Il’ichev, A.N., Matyshak, V.A., and Korchak, V.N., Kinet. Catal., 2015, vol. 56, no. 1, p. 115.
Venkataswany, P., Jampaiah, D., Aniz, C.U., and Reddy, B.M., J. Chem. Sci., 2015, vol. 127, no. 8, p. 1347.
Kim, H.J., Jang, M.G., Shin, D., and Han, J.W., ChemCatChem, 2020, vol. 12, p. 11.
Singhania, A., Ind. Eng. Chem. Res., 2017, vol. 56, no. 46, p. 13 594.
Malyutin, A.V., Liberman, E.Yu., Mikhailichenko, A.I., Avetisov, I.Kh., Koshkin, A.G., and Kon’kova, T.V., Katal. Prom-sti., 2013, no. 3, p. 54.
Guo, X., Qiu, Z., Mao, Z.Q., and Zhou, R., Phys. Chem. Chem. Phys., 2018, vol. 20, no. 40, p. 25 983.
Zhao, Z., Wang, R., Zhao, Q., Wang, E., Su, H., and Zeng, S., Adv. Mater. Res., 2013, vol. 773, p. 601.
Il’ichev, A.N., Firsova, A.A., and Korchak, V.N., Kinet. Catal., 2006, vol. 47, no. 4, p. 585.
Powder Diffraction Fale. Alphabetical Indexes. Inorganic phases, JCPDS, Pennsylvania: International Center for Diffraction Data, 1983.
Mirkin, L.I., Spravochnik po rentgenostrukturnomu analizu polikristallov (Handbook on X-ray Structural Analysis of Polycrystals), Moscow: Gosudarstvennoe izdatel’stvo fiziko-matematicheskoi literatury, 1961.
Tret’yakov, I.I., Shub, B.R., and Sklyarov, A.V., Zh. Fiz. Khim., 1970, vol. 44, p. 2112.
Handbook of Preparative Inorganic Chemistry, Brauer, G., Ed., Amsterdam: Elsevier, 1985, vols. 2–3.
Narula, C.K., Haack, L.P., Chun, W., Jen, H.-W., and Graham, G.W., J. Phys. Chem. B, 1999, vol. 103, p. 3634.
Firsova, A.A., Ilichev, A.N., Khomenko, T.I., Gorobinskii, L.V., Maksimov, Yu.V., Suzdalev, I.P., and Korchak, V.N., Kinet. Catal., 2007, vol. 48, no. 2, p. 282.
Fornasiero, P., Balducci, G., Monte, R.D., Kaspar, J., Sergo, V., Gubitosa, G., Ferrero, A., and Graziani, M., J. Catal., 1996, vol. 164, p. 173.
Manzoli, M., Monte, R.D., Boccuzzi, F., Coluccia, S., and Kaspar, J., Appl. Catal., B, 2005, vol. 61, p. 192.
Luo, M.F., Ma, J.-M., Lu, J.-Q., Song, Y.-P., and Wang, Y.-J., J. Catal., 2007, vol. 246, p. 52
Gomez-Cortes, A., Marquez, Y., Arenas-Alatorre, J., and Diaz, G., Catal. Today, 2008, vol. 133−135, p. 743.
Polster, C.S., Naier, H., and Baertsch, C.D., J. Catal., 2009, vol. 266, p. 308.
Moretti, E., Storaro, L., Talon, A., Lenarda, M., Riello, P., Frattini, R., Yuso, M.V.M., Jimenez-Lopez, A., Rodriguez-Gastellon, E., Ternero, F., Caballero, A., and Holgado, J.P., Appl. Catal., B, 2011, vol. 102, p. 627.
Arango-Diaz, A., Cecilia, J.A., Moretti, E., Talon, A., Nunez, P., Morrero-Jerez, J., Jimenez-Jimenez, J., Jimenez-Lopez, A., and Rodriguez-Gastellon, E., Int. J. Hydrogen Energy, 2014, vol. 39, p. 4102.
Wang, S.-P., Zheng, X.-C., Wang, X.-Y., Wang, S.-R., Zhang, S.-M., Yu, L.-H., Huang, W.-P., and Wu, S.-H., Catal. Lett., 2005, vol. 105, nos. 3−4, p. 163.
Matyshak, V.A. and Sil’chenkova, O.N., Kinet. Catal., 2019, vol. 60, no. 5, p. 573.
Ivanova, A.S., Kinet. Catal., 2009, vol. 50, no. 6, p. 797.
Krylov, O.V., Geterogennyi kataliz (Heterogeneous Catalysis), Mosvow: Akademkniga, 2004, p. 679.
Il’ichev, A.N., Bykhovskii, M.Ya., Fattakhova, Z.T., Shashkin, D.P., Matyshak, V.A., and Korchak, V.N., Kinet. Catal., 2018, vol. 59, no. 2, p. 179.
Skarman, B., Grandjean, D., Benfield, R., Hinz, A., Andersson, A., and Wallenberg, L. R., J. Catal., 2002, vol. 211, p. 119.
Funding
This work was carried out within the framework of a state contract of the Federal Agency for Scientific Organizations of Russia (V.46.13, 0082-2014-0007, project no. AAAA-A18-118020890105-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Abbreviations and designations: TPR-H2, temperature-programmed reduction with hydrogen; TPD, temperature-programmed desorption; BET, Brunauer–Emmett–Teller method; γ, conversion of CO, %; β, conversion of О2, %; \(N_{{{\text{C}}{{{\text{O}}}_{{\text{2}}}}}}^{{{\text{des}}}}{\text{,}}\) amount of desorbed CO2; \(N_{{{\text{CO}}}}^{{{\text{des}}}}{\text{,}}\) amount of desorbed CO; \(N_{{{\text{CO + C}}{{{\text{O}}}_{{\text{2}}}}}}^{{{\text{des}}}}{\text{,}}\) total amount of desorbed gas; L, crystallite size; Ssp, specific surface area; \({{N}_{{{{{\text{H}}}_{{\text{2}}}}}}}{\text{,}}\) total amount of absorbed hydrogen per square meter of oxide; Nc, calculated amount of hydrogen required for the reduction of copper oxide in the sample; \({{V}_{{{{{\text{H}}}_{{\text{2}}}}}}}{\text{,}}\) rate of oxygen consumption in reaction with hydrogen; and VCO, rate of oxygen consumption in reaction with carbon monoxide.
Rights and permissions
About this article
Cite this article
Il’ichev, A.N., Bykhovsky, M.Y., Fattakhova, Z.T. et al. Activity of 5% CuO/Ce1 – xPrxOy Catalysts in the Reaction of Carbon Monoxide Oxidation with Oxygen in an Excess of Hydrogen. Kinet Catal 62, 116–126 (2021). https://doi.org/10.1134/S0023158421010031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158421010031