Abstract
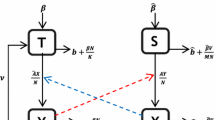
By extending a mechanistic model for the tick-borne pathogen systemic transmission with the consideration of seasonal climate impacts, host movement as well as the co-feeding transmission route, this paper proposes a novel modeling framework for describing the spatial dynamics of tick-borne diseases. The net reproduction number for tick growth and basic reproduction number for disease transmission are derived, which predict the global dynamics of tick population growth and disease transmission. Numerical simulations not only verify the analytical results, but also characterize the contribution of co-feeding transmission route on disease prevalence in a habitat and the effect of host movement on the spatial spreading of the pathogen.




Similar content being viewed by others
References
Allen LJS, Bolker BM, Lou Y, Nevei AL (2007) Asymptotic profiles of the steady states for an SIS epidemic patch model. SIAM J Appl Math 67(5):1283–1309
Arino J, Davis JR, Hartley D, Jordan R, Miller JM, van den Driessche P (2005) A multi-species epidemic model with spatial dynamics. Math Med Biol 22(2):129–142
Aronsson G, Kellogg RB (1978) On a differential equation arising from compartmental analysis. Math Biosci 38(1–2):113–122
Centers for Disease Control and Prevention. Recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html
Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J (1998) Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol 35(5):629–638
Dunn JM, Davis S, Stacey A, Diuk-Wasser MA (2013) A simple model for the establishment of tick-borne pathogens of Ixodes scapularis: a global sensitivity analysis of \({\cal{R}}_{0}\). J Theor Biol 335:213–221
Egyed L, Élö P, Sréter-Lancz Z, Széll Z, Balogh Z, Sréter T (2012) Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis 3(2):90–94
Fan G, Thieme HR, Zhu H (2015) Delay differential systems for tick population dynamics. J Math Biol 71(5):1017–1048
Gao D, Ruan S (2011) An SIS patch model with variable transmission coefficients. Math Biosci 232(2):110–115
Hancock PA, Brackley R, Palmer SCF (2011) Modelling the effect of temperature variation on the seasonal dynamics of Ixodes ricinus tick populations. Int J Parasitol 41(5):513–522
Heffernan JM, Lou Y, Wu J (2014) Range expansion of Ixodes Scapularis tick and of Borrelia Burgdorferi by migratory birds. Discrete Contin Dyn Syst Ser B 19(10):3147–3167
Hudson PJ, Norman RA, Laurenson MK, Newborn D, Gaunt M, Jones L, Reid H, Gould D, Bowers R, Dobson A (1995) Persistence and transmission of tick-borne viruses: Ixodes ricinus and louping-ill virus in red grouse populations. Parasitology 111(Suppl(S1)):49–58
Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Nicholas H (2006) Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4(9):660–669
Liu K, Lou Y, Wu J (2017) Analysis of an age structured model for tick populations subject to seasonal effects. J Differ Equ 263(4):2078–2112
Lou Y, Wu J, Wu X (2014) Impact of biodiversity and seasonality on Lyme-pathogen transmission. Theor Biol Med Model 11(1):50
Lou Y, Wu J (2017) Modeling Lyme disease transmission. Infect Dis Model 2(2):229–243
Nah K, Magpantay FMG, Bede-Fazekas Á, Röst G, Trájer AJ, Wu X, Zhang X, Wu J (2019) Assessing systemic and non-systemic transmission risk of tick-borne encephalitis virus in Hungary. PLoS ONE 14(6):e0217206
Nonaka E, Ebel GD, Wearing HJ (2010) Persistence of pathogens with short infectious periods in seasonal tick populations: the relative importance of three transmission routes. PLoS ONE 5(7):e11745
O’Connell S (2010) Lyme borreliosis: current issues in diagnosis and management. Curr Opin Infect Dis 23(3):231–235
Ogden NH, Lindsay LR, Morshed M, Sockett PN, Artsob H (2009) The emergence of Lyme disease in Canada. Can Med Assoc J 180(12):1221–1224
Ogden NH, Koffi JK, Lindsay LR, Fleming S, Mombourquett DC, Sanford C, Badcock J, Gad RR, Jain-Sheehan N, Moore S, Russell C, Hobbs L, Baydack R, Graham-Derham S, Lachance L, Simmonds K, Scott AN (2015) Surveillance for Lyme disease in Canada, 2009 to 2012. Can Commun Dis Rep 41(6):132–145
Ostfeld R (2010) Lyme disease: the ecology of a complex system. Oxford University Press, New York
Pettersson JH, Golovljova I, Vene S, Jaenson TG (2014) Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in northern Europe with particular reference to Southern Sweden. Parasite Vector 7(1):102
Randolph SE, Rogers DJ (1997) A generic population model for the African tick Rhipicephalus appendiculatus. Parasitology 115:265–279
Rosà R, Pugliese A, Norman R, Hudson PJ (2003) Threshoulds for disease persistence in models for tick-borne infections including non-viraemic transmission, extended feeding and tick aggregation. J Theor Biol 224(3):359–376
Rosà R, Pugliese A (2007) Effects of tick population dynamics and host densities on the persistence of tick-borne infections. Math Biosci 208(1):216–240
Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems. AMS ebooks Program 41(5):174
Torina A, Villari S, Blanda V et al (2020) Innate immune response to tick-borne pathogens: cellular and molecular mechanisms induced in the hosts. Int J Mol Sci 21(15):5437
Voordouw MJ (2015) Co-feeding transmission in Lyme disease pathogens. Parasitology 142(2):290–302
Wang W, Mulone G (2003) Threshold of disease transmission in a patch environment. J Math Anal Appl 285(1):321–335
Wang W, Zhao X-Q (2008) Threshold dynamics for compartmental epidemic models in periodic environments. J Dyn Differ Equ 20(3):699–717
Weng P, Zhao X-Q (2011) Spatial dynamics of a nonlocal and delayed population model in a periodic habitat. Discrete Contin Dyn Syst Ser A 29(1):343–366
Wu X, Magpantay FMG, Wu J, Zou X (2015) Stage-structured population systems with temporally periodic delay. Math Methods Appl Sci 38(16):3464–3481
Zhao X-Q (2012) Global dynamics of a reaction and diffusion model for Lyme disease. J Math Biol 65(4):787–808
Zhao X-Q (2017) Dynamical systems in population biology. Springer, New York
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 11701072 and 12071393). We are very grateful to the anonymous referees for careful reading and valuable comments that significantly improve our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
We show parameter values used in Fig. 5, including recruitment rates of larval ticks \(\rho _k\), probability of co-feeding transmission \(\eta _k\), systemic transmission probability between infected rodents and susceptible larvae \(\zeta _k^L\) and between susceptible rodents and infected nymphs \(\zeta _k^H\), and initial rodent densities \(H_k(0)\) in Table 3. Host migration proportions from patch j to patch k take the form \(m_{kj}(t)=(m_{kj}^{(1)}~m_{kj}^{(2)})(1 ~ \cos (2\pi t/365))^T\), \(j,k=1,2,\ldots ,9\). Table 4 lists all the components \(m_{kj}^{(1)}\) and \(m_{kj}^{(2)}\) of host migration proportions. Other parameters among the 9 patches are fixed at the same values as follows:
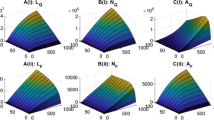
The comparison of accumulated infected nymph density for model (3) in a year: a 9 patches are isolated from each other; b host population can move freely among 9 patches
Without migration, we calculate the net reproduction numbers, the basic reproduction numbers and accumulated infected nymphal densities in 1 year for each patch k, \(k=1,2,\ldots , 9\) and list them in Table 5. When host migration is considered, the added numbers of accumulated yearly nymphal ticks and infected nymphal ticks across 9 patches are summarized in Table 6.
Rights and permissions
About this article
Cite this article
Zhang, X., Sun, B. & Lou, Y. Dynamics of a periodic tick-borne disease model with co-feeding and multiple patches. J. Math. Biol. 82, 27 (2021). https://doi.org/10.1007/s00285-021-01582-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01582-6
Keywords
- Tick-borne disease
- Patch model
- Co-feeding transmission
- Net reproduction number
- Basic reproduction number
- Global stability