Abstract
Magnetism of pure electrons is fundamental for understanding diverse magnetic phenomena in condensed matters but has not been fully investigated in experiments due to the lack of a tractable model system. Such an exotic material necessitates an exclusive magnetic interaction of electrons being devoid of orbital and lattice degrees of freedom. Here, we report the two-dimensional mixed-cation [YGdC]2+∙2e− electride, showing ferrimagnetic nature from the direct exchange interaction of magnetic interstitial electrons in interlayer space. We identify that magnetic interstitial electrons are periodically localized in octahedral and tetrahedral cavities between 2D cationic Y2−xGdx arrays. The mixed configuration of non-magnetic and magnetic cations in cavities induces divergent spin states and interactions of magnetic interstitial electrons, in which their direct exchange interaction overwhelms the interactions with magnetic cations, triggering the ferrimagnetic spin-alignment. This discovery facilitates further exploration of magnetic electrides and nurtures the study of two-dimensional magnetism of layered crystals and electron phases.
Similar content being viewed by others
Introduction
Understanding the nature of magnetic interaction in the pure electron systems has been of fundamental importance in many-body physics, giving a basic knowledge for the magnetism of condensed matters1,2,3,4,5,6. So far, many theoretical studies in the wide range of pure electron systems from the classical plasma to crystal have predicted that the spin-polarized electron phase inevitably emerges in the vicinity of Wigner crystallization7,8,9, but an experimentally accessible system has not been found yet. Indeed, the magnetic interaction of electrons in real materials has exclusively limited to the orbital electrons10,11,12,13, not isolated electrons from the electrostatic attraction of the atomic nucleus, provoking the only theoretical perspective for the magnetism of pure electrons. In this regard, the electrides, in which anionic electrons are localized at the interstitial space in a crystal lattice, not at the orbitals of constituent elements14,15,16,17,18, can be considered as a platform to study the inherent magnetism of electrons.
Spin states of interstitial electrons in electrides and their magnetic interactions have been theoretically proposed. It is predicted that the strongly localized interstitial anionic electrons (IAEs) in potassium electride under high-pressure exhibit a Stoner-type instability, leading to the ferromagnetism (FM)19. Notably, the recent experimental discovery of magnetic two-dimensional (2D) electrides reveals that the localized IAEs in interlayer space possess their own magnetic moments, playing as a key ingredient of the magnetism20,21,22. However, because the s-nature of magnetic IAEs are strongly hybridized with the orbital electrons of magnetic cations in positively charged layers, a direct interaction between magnetic IAEs in 2D electron layers remains elusive. Considering that the magnetic IAEs are localized in a 2D potential well of positively charged layers, their spin states can be manipulated to have a direct exchange interaction by enhancing the magnetic instability in the 2D cationic array. This can allow an exotic magnetism of interstitial electrons in the 2D free space of the electride crystals, substantiating the predicted magnetism of pure electron systems6,23. In this work, we design the 2D mixed-cation [Y2−xGdxC]2+∙2e− electride, in which an emergent magnetism of interstitial electrons evolves by their direct exchange interaction within 2D space, showing the unprecedented ferrimagnetic spin-alignment.
Results and Discussion
Mixed-cation electrides
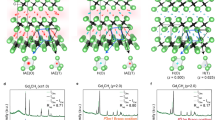
Figure 1a–d schematically illustrate the crystal structures of mixed-cation [Y2−xGdxC]2+∙2e− and binary [Re2C]2+∙2e− electrides (Re = Y or Gd)20,22, noting that the IAEs in 2D electron layers (blue layers in Fig. 1a, b) are localized in the periodic cavities of the octahedron (Fig. 1c) and tetrahedron (Fig. 1d), having different spin states and magnetic moments. These 2D electrides are crystallized in an anti-CdCl2-type layered structure with a space group of R\(\overline 3\)m (Fig. 1a), which has 2D electron layers between positively charged layers of [Y2−xGdxC]2+ and [Re2C]2+, preserving the charge neutrality of the system. The cationic slab is composed of edge-sharing octahedral units constructed by covalent bonding between Re and C (gray structures in Fig. 1a, b). It is noted that the interlayer space occupied by the IAEs is periodically disassembled into three cavities according to their electron density: (1) one six-coordinate octahedral cavity with a high-density IAEs and (2) two four-coordinate tetrahedral cavities with a low-density IAEs. More importantly, these three cavities have a different spin state in terms of direction and moment, as will be discussed in the following sections.
a−d Layered crystal structures of mixed-cation [Y2−xGdxC]2+·2e− (a) and rare-earth binary [Re2C]2+·2e− (Re = Y or Gd) electrides (b). Edge-sharing CY6−xGdx octahedral units are highlighted in gray color. IAE (O) and IAE (T) are the interstitial electrons confined in the octahedral (c) and tetrahedral (d) cavities, respectively. Red and blue arrows indicate spin-up and spin-down states of IAEs, respectively. IAE (O) has a majority spin-up state (c), whereas IAE (T) can have both minority spin-up and minority spin-down states (d). The IAEs exhibit the spin-alignments of ferrimagnetic nature in [Y2−xGdxC]2+·2e−, super-PM in [Y2C]2+·2e− and FM in [Gd2C]2+·2e−, respectively. e Lattice parameters of [Y2−xGdxC]2+·2e− obtained by the Rietveld refinements of the XRD data (Supplementary Fig. 1 and Table 1). Details of structural parameters are provided in Supplementary Fig. 2. f Actual Gd contents versus nominal Gd contents. Actual Gd contents are obtained from the ICP–MS (Supplementary Table 2) and Rietveld analysis. g, h XRD patterns of 2θ (g) and ϕ (h) scans of the cleaved single crystal [YGdC]2+·2e−, respectively. XRD patterns of [Re2C]2+·2e− also provided in g as references. Inset figures represent the picture of the cleaved surface (g) and STEM images (h) of single crystal sample. For STEM images, both the annular bright-field (ABF) and the high-angle annular dark-filed (HAADF) images of (010) plane are displayed.
The mixed-cation [Y2−xGdxC]2+∙2e− electride, is successfully synthesized in the form of primary solid solution (Fig. 1e), showing a monotonic increase of lattice parameter c with increasing x in Y2−xGdx cationic ratio due to the larger ionic size of Gd3+ (0.94 Å) than Y3+ (0.90 Å), whereas the lattice parameter a remains almost constant. The change of interlayer distance alters the localization degree of IAEs (Supplementary Fig. 2) and substantially affects the physical properties of the mixed-cation electrides such as electrical and magnetic properties18,21. The coincidence between nominal and actual compositions of the Y2−xGdx cationic ratio, which is confirmed from both the Rietveld refinement of X-ray diffraction (XRD) patterns and the inductively coupled plasma–mass spectrometry (ICP–MS) analysis (Fig. 1f), promises a systematic analysis of physical properties depending on the atomic arrangement in cationic layers rather than the theoretical density of anionic electrons (2.94 × 1022 cm−3 of [Y2C]2+∙2e− and 2.83 × 1022 cm−3 of [Gd2C]2+∙2e−). To identify the effect of 2D cationic Y2−xGdx array in the mixed-cation electrides and their intrinsic properties, we synthesized a representative composition of [YGdC]2+∙2e− in a single crystalline form by the floating zone (FZ) melting method and theoretically explored its magnetic structure in different configurations of Y and Gd (Supplementary Fig. 3). High crystallinity of the single crystal is verified by the exclusive (00 l) peaks in the 2θ scan of an as-cleaved crystal (Fig. 1g) and the three-fold symmetry of a rhombohedral structure in the ϕ scan (Fig. 1h). We also confirmed the well-defined layer structure of the proposed crystal structure using scanning transmission electron microscopy (STEM), which reveals the constant intensity at atomic columns of cations (inset of Fig. 1h).
Ferrimagnetic nature
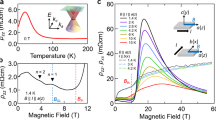
According to the ratio of non-magnetic and magnetic cations in the 2D cationic array, the mixed-cation [Y2−xGdxC]2+∙2e− electrides show a diverse range of magnetic properties. Two end members of binary electrides, [Y2C]2+∙2e− (x = 0) and [Gd2C]2+∙2e− (x = 2) show typical behaviors of paramagnetic (PM) and FM materials, respectively20,22. However, the mixed-cation electrides (0 < x < 2) exhibit a distinct feature from both PM and FM characters. As shown in Fig. 2a, both the smooth increasing behavior around Curie temperature (TC) and non-saturated feature with a slight decrease upon a cooling hint that the magnetic properties of mixed-cation electrides are attributed to the ferrimagnetic or spin-glass states, which may be induced by the magnetic instability in the atomic configuration of 2D cationic Y2−xGdx arrays. In addition, the systematic increases of three magnetic parameters, (1) saturation magnetic field (Hs, inset in Fig. 2b), (2) TC (black dots in Fig. 2c), and (3) effective magnetic moment (μeff., red dots in Fig. 2c), suggest that the mixed configuration of Y and Gd cations is probably responsible for the magnetic properties. Meanwhile, the negligible difference between zero-field cooling and field cooling curves for the sample of x ~ 1 undoubtedly rules out the possibility of the spin-glass formation (Supplementary Fig. 6). On the contrary, all deviations from the Curie–Weiss law (arrows in Fig. 2d) for each mixed-cation electride prove the ferrimagnetic nature, where a clear deviation appears in the sample of x ~ 1. However, as an intermediate magnetic state between PM [Y2C]2+∙2e− and FM [Gd2C]2+∙2e− electrides, the FM or weak-FM ordering is fairly anticipated in the mixed-cation electrides. Thus, the result that abnormal ferrimagnetic characters are induced by the mixed Y and Gd arrangement remains elusive, demanding the identification of the role of IAEs for the ferrimagnetic ordering.
a Temperature dependence (T) of magnetization (M) under the DC applied magnetic field (HDC) of 0.1 T. A smaller HDC than Hs is chosen so as to stress the ferrimagnetic character (Supplementary Fig. 4). b HDC dependence of M at 2 K. Inset shows Gd content (x) dependent Hs. c TC (black dots) and μeff. (red dots) of [Y2−xGdxC]2+·2e− as a function of Gd content (x). The black and red dashed lines are guides to show the monotonical changes of the TC and μeff. values. The first-derivative plots of M–T curves to determine the TC are provided in Supplementary Fig. 5. The μeff. values are obtained from the saturation magnetization in b. d M−1–T plots. Black dashed lines are Curie–Weiss fits. The arrows indicate the deviations from the Curie–Weiss law for each sample. The most significant deviation is found for x = 0.93 (Enlarged figure is seen in Supplementary Fig. 6b).
By examining the anisotropy of magnetic properties in the single crystal [YGdC]2+∙2e− electride, we addressed the origin of ferrimagnetic properties of mixed-cation electrides. It is noted that the saturation magnetic moments of the single crystal [YGdC]2+∙2e− (~7.7 μB) and [Gd2C]2+∙2e− (~15.6 μB) are larger than that (~7.6 μB) of Gd metal (Fig. 3a). The differences from the magnetic moment of Gd metal are not of extrinsic factors such as magnetic impurities, as revealed by ICP–MS measurements (Supplementary Table 3)22, concluding that the excess magnetic moments come from the inherent contributions of IAEs. However, comparing the excess magnetic moments of IAEs of [YGdC]2+∙2e− and [Gd2C]2+∙2e−, smaller excess magnetic moment (~0.1 μB) of [YGdC]2+∙2e− than that (0.4 μB = 15.6 − 7.6 × 2 (two moles of Gd)) of FM [Gd2C]2+∙2e− implies that the spin alignment of IAEs in [YGdC]2+∙2e− is less parallel than the FM aligned IAEs in [Gd2C]2+∙2e−. Considering that the interlayer space of [YGdC]2+∙2e− is smaller than that of [Gd2C]2+∙2e− (Supplementary Fig. 2b), the difference in the excess magnetic moment between these two electrides is not attributed to the chemical pressure from the positively charged cationic layers21. We also note that the FM signal at TC ~ 220 K appears for both directions, but a substantial magnetic suppression below T* ~ 165 K occurs in the only in-plane direction (Fig. 3b). This magnetic suppression originates from the existence of negative exchange interaction along the in-plane direction, verifying the ferrimagnetic nature.
a HDC dependences of anisotropic μeff. for single crystalline [YGdC]2+·2e− and [Gd2C]2+·2e− samples. The μeff. of Gd metal is also provided as a reference. The lower Hs of in-plane direction indicates that the magnetic easy-plane develops along the in-plane direction. b T dependences of anisotropic Μ along in-plane and out-of-plane directions. Inset figures are schematics to represent the reinforcement of exchange interaction of IAEs along the direction of the applied HDC (violet arrows). c Imaginary part of AC magnetization (Μ “) along the in-plane direction under the various frequencies at AC applied magnetic field (HAC) of 1 Oe. The DC applied field is set to zero for these measurements. Inset figure shows the frequency (f) dependences of TC and T*. T* denotes the onset temperature when the magnetization starts to suppress. The real part of AC magnetization data (Μ ‘) is provided in Supplementary Fig. 7. d T dependence of anisotropic ρ for [YGdC]2+·2e− and [Gd2C]2+·2e−. Transitions around TC of 220 K for [YGdC]2+·2e− and 350 K for [Gd2C]2+·2e− are indicated by arrows. The residual resistivity ratios of [Y2−xGdxC]2+·2e− and conduction electron density (CED) of both [YGdC]2+·2e− and [Gd2C]2+·2e− are shown in Supplementary Figs. 9 and 10, respectively.
The ferrimagnetism of [YGdC]2+∙2e− is explicitly proved by the AC magnetization measurements, showing a magnetic suppression in both real and imaginary parts below T* (Fig. 3c and Supplementary Fig. 7). Because the spin-glass state shows the frequency-dependent freezing temperature shift in the temperature-dependent AC magnetization data24, neither frequency dependence of T* nor TC shift completely excludes the spin-glass formation (inset of Fig. 3c). A single oxidation state of the Gd 4 f core level, which indicates the existence of the only Gd3+ state (Supplementary Fig. 8), further supports that the ferrimagnetic ordering in [YGdC]2+∙2e− originates from magnetic IAEs, not FM Gd cations. Furthermore, this ferrimagnetic nature is strongly correlated with the electrical properties with a negligible anisotropy compared to the strong anisotropy of FM [Gd2C]2+∙2e−. The decreased resistivity (ρ) in the out-of-plane direction for [YGdC]2+∙2e− compared to that of [Gd2C]2+∙2e− (Fig. 3d) can be ascribed to the enhanced probability of wavefunction overlap of conduction electrons by the lattice shrinkage along the c-axis (Fig. 1e). In contrast, the ρ along the in-plane direction is largely increased due to the scattering of conduction electrons with the ferrimagnetically aligned spins of IAEs rather than the structural factor (almost constant a), giving a considerably smaller anisotropic ratio of ~2 for ferrimagnetic [YGdC]2+∙2e− than ~60 for FM [Gd2C]2+∙2e−.
Interacting magnetic interstitial electrons
To elucidate how the magnetic IAEs induce the ferrimagnetic ground state in FM element contained [YGdC]2+∙2e−, the theoretical considerations are made in the viewpoint of the magnetic exchange interaction between IAEs having divergent spin states in polyhedral cavities. As shown in Fig. 4a, the interlayer space between the 2D cationic arrays of positively charged layers can be disassembled into the periodic polyhedral cavities of the tetrahedron (T)–octahedron (O)–tetrahedron (T) according to electron density and the spin state of IAEs. In particular, for the ferrimagnetic [YGdC]2+∙2e−, we consider two possible combinations of the polyhedral cavities with different configurations of Y and Gd cations: Y3Gd–Y3Gd3–YGd3 (type 1) and Y2Gd2–(Y2Gd4 or Y4Gd2)–Y2Gd2 (type 2). From the calculated electron localization functions (ELFs)25 and magnetization density maps (MDMs) of two ferrimagnetic [YGdC]2+∙2e− and FM [Gd2C]2+∙2e− (Fig. 4b–d), it is revealed that all the localized IAEs in cavities have their own magnetic moments, indicating the quasi-atomic nature of magnetic electrons. The positive valence states of Gd and Y cations estimated by Bader charge analysis indicate that the electrons at the interstitial site are mainly transferred from the valence orbitals of Y and Gd elements (Supplementary Table 4). The highly concentrated IAEs are commonly found in the octahedral cavities, IAE(O), exhibiting majority spin-up states, which are parallel to the spins of Gd 4f electrons. On the other hand, the minority spins of less concentrated IAEs always emerge in the tetrahedral cavities, IAE(T), and their spin states are strongly dominated by the number of Gd cations in polyhedral. The minority spin-up state appears in Gd-rich (YGd3) tetrahedral cavities; however, it is noted that the minority spin-down state emerges in the Gd-poor cavities of Y3Gd (type 1) and Y2Gd2 (type 2). These divergent spin states of IAEs in periodic cavities are responsible for the observed ferrimagnetism of mixed cation electrides.
a Periodic polyhedral cavities trapping IAEs in the 2D interlayer space between cationic [YGdC]2+ layers. Two possible configurations (type 1 and type 2) of mixed-cations in the periodic polyhedral cavities are shown. Exchange interactions of Je–e and Je–Gd are represented by red and green zigzag lines, respectively. There is a negligible magnetic exchange interaction between Y cations and IAEs due to the non-magnetic character. b–d ELFs and MDMs for [YGdC]2+·2e− (b, c) and [Gd2C]2+·2e− (d). Black circles denote the maximum points in ELFs. Colored lines in b–d are the distances between black circles. Color of the MDM for each IAE determines the spin direction. Detailed electronic structures are shown in Supplementary Fig. 11. e Spin dynamics of the magnetic IAEs. Two regions divide the ferrimagnetic (sky-blue) and FM (pink) orderings of IAEs. The distance of IAE (O)–IAE (T) is plotted as a function of the cationic ratio in the tetrahedral cavities. The spin direction of IAE (T) strongly depends on the inter-distance of magnetic IAEs.
Most of all, in contrast to the distance between IAEs in FM [Gd2C]2+∙2e−, we note that the distance between divergent IAEs in central octahedral and neighboring tetrahedral cavities with majority spin-up and minority spin-down states is reduced in ferrimagnetic [YGdC]2+∙2e−. It is clearly shown in the ELF plots that the distance between IAE(O) with spin-up and IAE(T) with spin-down in two types of ferrimagnetic [YGdC]2+∙2e− is remarkably reduced down to ~1.9 Å by ~15% than the distance (~2.2 Å) between IAEs with same spin-up states in FM [Gd2C]2+∙2e−, which strongly suggests that the direct electron-electron exchange interaction (Je–e) develops between the magnetic IAEs. The evolution of the antiparallel spin pair of IAE(O)–IAE(T) in the reduced distance is well described by the pair distribution function of the spin-polarized pure electron system in which the antiparallel spin alignment of electrons is more favorable than the parallel spin alignment when the distance between electrons is decreased6,23 (Fig. 4e). When the exchange interaction between IAE and nearby Gd cation (Je–Gd) becomes weaker in the Gd-poor tetrahedral cavities, the minority IAE(T) undergoes the spin-flip in the reduced distance with IAE(O), in contrast to the unchanged spin-up state and distance with IAE(O) of minority IAE(T) in the Gd-rich tetrahedral cavities. Finally, by the design of hydrogen-substituted [YGdC]2+∙e−∙H− (Supplementary Fig. 12), it is clarified that the direct Je–e between IAEs overwhelms the Je–Gd between IAE(T) and Gd cations, leading to the spin-flip of the IAEs. Indeed, neither the distance reduction nor the spin-down state is observed in the [YGdC]2+∙e−∙H−, where the Je–e disappears by the replacement of majority spin-up IAE(O) with the non-magnetic H−(O). Thus, we conclude that the emergence of the unprecedented ferrimagnetic spin-alignment in the mixed-cation [YGdC]2+∙2e− is triggered by the superior Je–e among divergent exchange interactions of magnetic IAEs.
Discussion
In summary, the direct magnetic interaction between interstitial electrons in 2D free space is discovered by the experimental design of mixed-cation electride crystal. The various magnetic properties in 2D electrides, which highlight the present ferrimagnetism together with FM, antiferromagnetism, and super-PM of previous 2D electrides, indicate that the magnetic IAEs can have an inherent electron-electron exchange interaction as well as interactions with magnetic elements in the cationic framework. Our findings pave the way towards a pure magnetic electron phase utilizing the magnetic interstitial electrons as a magnetically active elemental particle.
Methods
Sample preparation
All sample preparations were performed in the glove box (H2O < 1 ppm, O2 < 1 ppm) filled with purified Ar gas (99.999%) because the samples are easily oxidized in the ambient atmosphere. The Y and Gd metals and graphite pieces were mixed in a stoichiometric molar ratio, and the mixture is melted by the arc-melting method. The melting process was repeated at least three times to obtain homogeneity of the polycrystalline [Y2−xGdxC]2+∙2e− electrides. For the single crystal growth, the FZ melting method was used. The synthesized polycrystalline samples were used to prepare the feed and seed rods. The feed and seed rods were rotated in opposite directions at the same speed of 6 rpm. A growth speed was limited below 4 mm per hour due to the low viscosity of melted [YGdC]2+∙2e− electride.
Crystal structure analysis
All measurements were carried out under the Ar atmosphere. The XRD measurements were performed by D/MAX-2500/PC, Rigaku analyzing with Cu Kα radiation (λ = 1.5418 Å). The samples were placed in the Ar-filled sample holder to prevent oxidization during the measurements. The Rietveld refinements were carried out using the Generalized Structure and Analysis System26. For the compositional analysis, ICP–MS was performed using 7900 ICP–MS (Agilent). For the STEM measurements, a dual-beam focused ion beam (FIB) system was used to prepare the sample. FIB process was performed using 15.0 kV of acceleration voltage. After the FIB process, samples were placed on the lift-out grid. STEM measurements were performed with a probe Cs-corrected JEM-ARM 200 F (JEOL), which was operated mostly at 80 kV of acceleration voltage. The X-ray photoelectron spectroscopy (XPS) for the single crystal [YGdC]2+∙2e− electride was carried out using an R400 spectrometer (VG Scienta) with the X-ray source of monochromatic Al Kα (1486.7 eV). The base pressure of the XPS measurements was ~ 5.0 × 10−10 Torr.
Characterization of physical properties
For the electrical and magnetic properties measurements, the sample was prepared in glove boxes with the purified Ar gas (99.999%). The four-point probe configuration of the electrical contacts was made on the flat surfaces of the single crystal [YGdC]2+·2e− and polycrystalline [Y2−xGdxC]2+·2e− electrides using silver epoxy. The sample was covered using Apiezon N grease to prevent the sample oxidation. For the magnetic property measurements, a vibrating sample magnetometer was used. The μeff. is calculated by μeff. = μsat./N⋅μB, where μsat. is the saturation magnetic moment at 2 K, N is the number of elements, and μB is the Bohr magneton.
Electronic and magnetic structure calculations
First-principles density functional theory calculations were carried out using the projector augmented plane-wave method and the generalized gradient approximation with the Perder–Burke–Ernzerhof functional implemented in the Vienna ab initio simulation program code27,28,29. The plane-wave-basis cut-off energy was set to 600 eV. The 4 f, 5 s, 5p, 5d, and 6 s electrons of Gd, the 4 s, 4p, 4d, and 5 s electrons of Y, and the 2 s and 2p electrons of C are considered as valence electrons. On-site Coulomb interaction values of U = 7.7 eV and J = 0.7 eV were used for the Gd 4 f electrons30. Self-consistency was carried out using a 2a × b × c supercell containing 18 atoms with a 2 × 4 × 1 k-point mesh. The initial magnetic moments of each element were set as Y = 0 μB, Gd = 7 μB, and C = 0 μB in accordance with the experimentally observed magnetic properties of FM [Gd2C]2+∙2e− and PM [Y2C]2+∙2e− electrides20,22. The obtained magnetic moments after structural and electronic relaxations are provided in Supplementary Table 5. Structural relaxation was performed until the Hellmann-Feynman forces for all elements were less than 5 × 10−5 eV Å−1. The MDMs were calculated from the difference in charge density between spin-up and spin-down components. The crystal structures, ELFs, and MDMs were visualized using the Visualization for Electronic and Structural Analysis code31. The atomic charges were estimated from a Bader charge analysis32.
Data availability
The data that support the plots in this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
White, R. M. Quantum Theory of Magnetism. (Springer, 1983).
Nolting, W. & Ramakanth, A. Quantum Theory of Magnetism. (Springer, 2009).
Burch, K. S., Mandrus, D. & Park, J.-G. Magnetism in two-dimensional van der Waals materials. Nature 563, 47–52 (2018).
Hwang, H. Y. et al. Emergent phenomena at oxide interfaces. Nat. Mater. 11, 103–113 (2012).
Tokura, Y., Kawasaki, M. & Nagaosa, N. Emergent functions of quantum materials. Nat. Phys. 13, 1056–1068 (2017).
Sólyom, J. Fundamentals of the Physics of Solids: Volume 3-Normal, Broken-Symmetry, and Correlated Systems. (Springer, 2010).
Ceperley, D. M. & Alder, B. J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 45, 566–569 (1980).
Attaccalite, C., Moroni, S., Gori-Giorgi, P. & Bachelet, G. B. Correlation energy and spin polarization in the 2D electron gas. Phys. Rev. Lett. 88, 256601 (2002).
Cândido, L., Bernu, B. & Ceperley, D. M. Magnetic ordering of the three-dimensional Wigner crystal. Phys. Rev. B 70, 094413 (2004).
Young, D. P. et al. High-temperature weak ferromagnetism in a low-density free-electron gas. Nature 397, 412–414 (1999).
Ceperley, D. Return of the itinerant electron. Nature 397, 386–387 (1999).
Shashkin, A. A., Kravchenko, S. V., Dolgopolov, V. T. & Klapwijk, T. M. Indication of the ferromagnetic instability in a dilute two-dimensional electron system. Phys. Rev. Lett. 87, 086801 (2001).
Horsch, P., Sofin, M., Mayr, M. & Jansen, M. Wigner crystallization in Na3Cu2O4 and Na8Cu5O10 chain compounds. Phys. Rev. Lett. 94, 076403 (2005).
Dye, J. L. Electrides: ionic salts with electrons as the anions. Science 247, 663–668 (1990).
Dye, J. L. Electrons as anions. Science 301, 607–608 (2003).
Matsuishi, S. et al. High-density electron anions in a nanoporous single crystal: [Ca24Al28O64]4+(4e−). Science 301, 626–629 (2003).
Kim, S. W. & Hosono, H. Synthesis and properties of 12CaO·7Al2O3 electride: review of single crystal and thin film growth. Philos. Mag. 92, 2596–2628 (2012).
Lee, K., Kim, S. W., Toda, Y., Matsuishi, S. & Hosono, H. Dicalcium nitride as a two-dimensional electride with an anionic electron layer. Nature 494, 336–340 (2013).
Pickard, C. J. & Needs, R. J. Predicted pressure-induced s-band ferromagnetism in alkali metals. Phys. Rev. Lett. 107, 087201 (2011).
Park, J. et al. Strong localization of anionic electrons at interlayer for electrical and magnetic anisotropy in two-dimensional Y2C electride. J. Am. Chem. Soc. 139, 615–618 (2017).
Park, J. et al. Tuning the spin-alignment of interstitial electrons in two-dimensional Y2C electride via chemical pressure. J. Am. Chem. Soc. 139, 17277–17280 (2017).
Lee, S. Y. et al. Ferromagnetic quasi-atomic electrons in two-dimensional electride. Nat. Commun. 11, 1526 (2020).
Martin, R. M. Electronic Structure: Basic Theory and Practical Methods (Cambridge University Press, 2004).
Anand, V. K., Adroja, D. T. & Hillier, A. D. Ferromagnetic cluster spin-glass behavior in PrRhSn3. Phys. Rev. B 85, 014418 (2012).
Silvi, B. & Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371, 683–686 (1994).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Abdelouahed, S., Baadji, N. & Alouani, M. Electronic structure and x-ray magnetic circular dichroism of gadolinium beyond the local spin density approximation. Phys. Rev. B 75, 094428 (2007).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 44, 1272–1276 (2011).
Sanville, E., Kenny, S., Smith, R. & Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 28, 899–908 (2007).
Acknowledgements
This work was supported by the Institute for Basic Science (IBS-R011-D1) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science, ICT & Future Planning) (No. 2015M3D1A1070639).
Author information
Authors and Affiliations
Contributions
S.W.K. conceived the idea and organized the research. S.Y.L. grew the single-crystals and fabricated all polycrystals. J.B., D.T., and S.-G.K. carried out computational studies. S.Y.L., B.I.Y., and H.Y.S. performed the transport and magnetic measurements. S.Y.L., J.B., Y.K., and K.L. analyzed the structural, electrical, and magnetic properties. All the authors discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.Y., Bang, J., Song, H.Y. et al. Mixed-cation driven magnetic interaction of interstitial electrons for ferrimagnetic two-dimensional electride. npj Quantum Mater. 6, 21 (2021). https://doi.org/10.1038/s41535-021-00319-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41535-021-00319-4