Abstract
Objective
To estimate the cost-effectiveness and value of information of cabozantinib compared to nivolumab in advanced renal cell carcinoma (RCC) patients, who previously failed treatment from a societal perspective in South Korea.
Methods
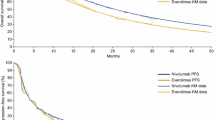
A partitioned survival model was used to evaluate the incremental cost-utility ratio (ICUR) of cabozantinib versus nivolumab. Overall survival (OS) and progression-free survival (PFS) curves were obtained from a network meta-analysis that included METEOR and CheckMate 025 trial results. Utility values for health states and adverse events were estimated based on the EQ-5D-5L data of METEOR trial with a Korean-specific tariff. Costs were estimated by a micro-costing approach using healthcare claims data and expert consultation. The impact of uncertainties in the model were explored by scenario analyses, and deterministic and probabilistic sensitivity analyses. The expected value of perfect information (EVPI) was estimated to assess the value of future research to decrease decision uncertainty.
Results
Compared to nivolumab, cabozantinib was associated with improved OS, PFS, and quality-adjusted life-years (QALYs) at greater cost. The ICUR was $34,445 per QALY. In sensitivity analysis, drug costs had the greatest influence on the ICUR. Cabozantinib had a 68.0% probability of being cost-effective at a threshold of 2 times gross domestic product (GDP) per capita. The population EVPI was $82.6 million at 2 GDP threshold.
Conclusions
Cabozantinib was found to be cost-effective for advanced RCC patients after failure of prior therapy at a 2 GDP threshold. Future research that costs less than the estimated population EVPI would be worth considering for a comparison of cabozantinib and nivolumab.





Similar content being viewed by others
References
Escudier B, Eisen T, Porta C, Patard J, Khoo V, Algaba F, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl_7):vii65–71.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Jung K-W, Won Y-J, Oh C-M, Kong H-J, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat Off J Korean Cancer Assoc. 2017;49(2):292.
Korean Urological Oncology Society. Clinical Guidelines on Renal Cell Carcinoma [Published in Korean]. 2015.
Kröger M, Menzel T, Gschwend J, Bergmann L. Life quality of patients with metastatic renal cell carcinoma and chemo-immunotherapy—a pilot study. Anticancer Res. 1999;19(2C):1553–5.
Litwin MS, Fine JT, Dorey F, Figlin RA, Belldegrun AS. Health related quality of life outcomes in patients treated for metastatic kidney cancer: a pilot study. J Urol. 1997;157(5):1608–12.
Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney cancer, version 2. 2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Net. 2017;15(6):804–34.
Powles T, Staehler M, Ljungberg B, Bensalah K, Canfield SE, Dabestani S, et al. European Association of Urology Guidelines for clear cell renal cancers that are resistant to vascular endothelial growth factor receptor-targeted therapy. 2016.
Zhou L, Liu X-D, Sun M, Zhang X, German P, Bai S, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Amzal B, Fu S, Meng J, Lister J, Karcher H. Cabozantinib versus everolimus, nivolumab, axitinib, sorafenib and best supportive care: a network meta-analysis of progression-free survival and overall survival in second line treatment of advanced renal cell carcinoma. PLoS ONE. 2017;12(9):e0184423.
Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl_5):v58–68.
Edwards SJ, Wakefield V, Cain P, Karner C, Kew K, Bacelar M, et al. Axitinib, cabozantinib, everolimus, nivolumab, sunitinib and best supportive care in previously treated renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess (Winchester, England). 2018;22(6):1.
Meng J, Lister J, Vataire A-L, Casciano R, Dinet J. Cost-effectiveness comparison of cabozantinib with everolimus, axitinib, and nivolumab in the treatment of advanced renal cell carcinoma following the failure of prior therapy in England. Clin Econ Outcomes Res CEOR. 2018;10:243.
Eckermann S, Karnon J, Willan AR. The value of value of information. Pharmacoeconomics. 2010;28(9):699–709.
Bindels J, Ramaekers B, Ramos IC, Mohseninejad L, Knies S, Grutters J, et al. Use of value of information in healthcare decision making: exploring multiple perspectives. Pharmacoeconomics. 2016;34(3):315–22.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: OUP; 2006.
Jutkowitz E, Alarid-Escudero F, Choi HK, Kuntz KM, Jalal H. Prioritizing future research on allopurinol and febuxostat for the management of gout: value of information analysis. Pharmacoeconomics. 2017;35(10):1073–85.
Health UD, Services H. Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute. 2009;4(03).
Data on file. CABOMETYXTM (cabozantinib) Health Economic Model. 14 Mar 2017. Ipsen Inc., Paris, France.
US Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 27 Mar 2019.
Ouwens MJ, Philips Z, Jansen JP. Network meta-analysis of parametric survival curves. Res Synth Methods. 2010;1(3–4):258–71.
Kim S-H, Ahn J, Ock M, Shin S, Park J, Luo N, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res. 2016;25(7):1845–52.
Health Insurance Review and Assessment Service. Handbooks of review and assessment guidelines for health services. 2016. http://www.hira.or.kr/bbs/bbsCDownLoad.do?apndNo=39611&apndBrdBltNo=2290&apndBrdTyNo=1&apndBltNo=68. Accessed 27 Mar 2019.
Korean Statistical Information Service (KOSIS). Statistical Database. http://kosis.kr/eng/. Accessed 27 Mar 2019.
Bae EY. Guidelines for economic evaluation of pharmaceuticals in Korea. J Prev Med Public Health. 2008;41(2):80–3.
Yim E-Y, Lim SH, Oh M-J, Park HK, Gong J-R, Park SE, et al. Assessment of pharmacoeconomic evaluations submitted for reimbursement in Korea. Value Health. 2012;15(1):S104–10.
You M-Y. Economic evaluation of new drug treatments and changes in drug price policies. J Korean Soc Health Syst Pharm. 2014;31(6):1044–53.
Stewart G, Eddowes L, Hamerslag L, Kusel J. The impact of NICE’s end-of-life threshold on patient access to new cancer therapies in England and Wales. Value Health. 2014;17(3):A6.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making#appraisal-of-the-evidence. Accessed 27 Mar 2019.
Swinburn P, Lloyd A, Nathan P, Choueiri TK, Cella D, Neary MP. Elicitation of health state utilities in metastatic renal cell carcinoma. Curr Med Res Opin. 2010;26(5):1091–6.
Graves A, Hessamodini H, Wong G, Lim WH. Metastatic renal cell carcinoma: update on epidemiology, genetics, and therapeutic modalities. Immunotargets Ther. 2013;2:73.
Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552–62.
Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015–2016. JAMA Intern Med. 2018;178(11):1451–7.
Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–28.
Porta C, Szczylik C, Casciano R, Fu S, Amzal B, Lister J, et al. Second-line cabozantinib versus nivolumab in advanced renal cell carcinoma: systematic review and indirect treatment comparison. Crit Rev Oncol Hematol. 2019;139:143–8.
Capri S, Porta C, Delea TE. Cost-effectiveness of pazopanib versus sunitinib as first-line treatment for locally advanced or metastatic renal cell carcinoma from an Italian national health service perspective. Clin Ther. 2017;39(3):567–80 (e2).
National Institute for Health and Care Excellence. Single Technology Appraisal-Cabozantinib for previously treated advanced renal cell carcinoma [ID931]. 2017. https://www.nice.org.uk/guidance/ta463/documents/committee-papers. Accessed 27 Mar 2019.
Health Insurance Review and Assessment Service, National Health Insurance Service. 2016 National Health Insurance Statistical Yearbook. 2017.
Korea Institute for Health and Social Affairs. The Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005-Health Service Utilization. 2006.
Korea Institute for Health and Social Affairs, National Health Insurance Service. The 2014 Korea Health Panel Survey. 2016.
Acknowledgements
This research was funded by Ipsen Korea. This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (Ministry of Science and ICT) (Grant number: NRF-2020R1F1A1069526). We used the Health Insurance Review and Assessment Service-National Patient Sample data 2016 (HIRA-NPS-2016-0092); however, we declare that the results do not reflect the positions of either the Health Insurance Review and Assessment Service or the Ministry of Health and Welfare in Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Ipsen Korea. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (grant number: NRF-2020R1F1A1069526).
Conflict of interest
Suh, H Kim, S Kim, and Han received research grants from Korea Health Industry Development Institute, Ministry of Food and Drug Safety, Ministry of Health and Welfare, National Research Foundation, Abbvie Korea, Amgen Inc, Amgen Korea, Handok-Teva, Ipsen Korea, and Pfizer Korea.
Ethics approval
This study was approved by the Institutional Review Board of Pusan National University (PNU IRB/2019_124_HR).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design, data collection, data analysis, and interpretation of results. The first draft of the manuscript was written by Siin Kim and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Most of the data generated or analyzed during this study are included in this published article and its supplementary information files. The cost-effectiveness model that supports the findings of this study is available from the corresponding author upon reasonable request and with permission of Ipsen Korea.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, S., Han, S., Kim, H. et al. Cost-Effectiveness and Value of Information of Cabozantinib Treatment for Patients with Advanced Renal Cell Carcinoma After Failure of Prior Therapy in South Korea. Appl Health Econ Health Policy 19, 545–555 (2021). https://doi.org/10.1007/s40258-021-00640-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-021-00640-w