Abstract
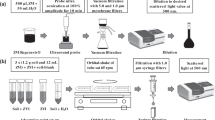
Strongly acid soils (pHKCl < 3.3) are widespread in the northeast of European Russia and are characterized by the low precision of potentiometric measurements of the components of exchangeable acidity. The goal of our study was to identify the causes that reduce the precision of measuring the exchangeable acidity of these soils using single- and two-component model systems of aluminum and iron(III) ions in concentrations similar to those in extracts from the strongly acid soils. Using model solutions, it was shown that the high acidity of salt extracts from soils is caused by the presence of iron(III) ions. The precision of measurements of metal ions was determined by the mode of stirring of the analyzed solution (continuous or discrete), as well as by the duration of interaction of the titrant with it. This was due to the presence of disperse systems in the initial model solutions. Particle parameters (hydrodynamic diameter D 70–90 nm, ζ-potential 19–30 mV) were determined by the dynamic light scattering and laser Doppler electrophoresis. The presence of other components—organic compounds and fine particles of silicates and iron hydroxides capable of passing through the blue ribbon filter pores in the salt extracts from soils—further reduces the precision of measuring the total Al3+ and Fe3+ content (exchangeable acidity) by the potentiometric method and casts doubt on the possibility of its application. When working with strongly acid soil samples, the use of atomic emission method is recommended.





Similar content being viewed by others
REFERENCES
E. V. Vanchikova, E. V. Shamrikova, N. V. Bespyatykh, E. V. Kyz”yurova, and B. M. Kondratenok, “Metrological assessment of the methods for measuring the contents of acids and ion metals responsible for the exchangeable acidity of soils,” Eurasian Soil Sci. 48 (2), 169–176 (2015). https://doi.org/10.1134/S1064229314120102
Yu. M. Vodyanitskii, Iron Compounds and Their Role in Soil Protection (Dokuchaev Soil Science Inst., Moscow, 2010) [in Russian].
E. V. Zhngurov and I. I. Golubeva, “Morphological characteristics and petrographic features of the minerals of the automorphic soils of Northern Timan,” Vestn. Inst. Geol., Komi Nauchn. Tsentr, Ural Otd., Ross. Akad. Nauk, No. 2 (182), 13–17 (2010).
E. V. Zhangurov, M. P. Lebedeva, and I. V. Zaboeva, “Microstructure of genetic horizons in automorphic soils of the Timan Ridge,” Eurasian Soil Sci. 44 (3), 261–271 (2011).
E. V. Zhangurov, V. D. Tonkonogov, and I. V. Zaboeva, “Automorphic soils of the central and southern Timan Ridge,” Eurasian Soil Sci. 41 (12), 1247–1255 (2008).
L. L. Shishov, V. D. Tonkonogov, I. I. Lebedeva, and M. I. Gerasimova, Classification and Diagnostic System of Russian Soils (Oikumena, Smolensk, 2004) [in Russian].
K. G. Kulikov and T. V. Koshlan, “Measurement of sizes of colloid particles using dynamic light scattering,” Tech. Phys. 60, 1758–1764 (2015).
IUSS Working Group WRB, World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, World Soil Resources Report No. 106 (US Food and Agriculture Organization, Rome, 2015; Moscow State Univ., Moscow, 2017).
D. L. Pinskii, Ion Exchange Processes in Soils (Pushchino, 1997) [in Russian].
A. V. Sokolov, Agrochemical Analysis of Soils (Nauka, Moscow, 1975) [in Russian].
T. A. Sokolova, “Low-molecular-weight organic acids in soils: sources, composition, concentrations, and functions: a review,” Eurasian Soil Sci. 53 (5), 580–594 (2020). https://doi.org/10.1134/S1064229320050154
T. A. Sokolova, I. I. Tolpeshta, and S. Ya. Trofimov, Soil Acidity. Acid-Base Soil Buffering. Aluminum Compounds in the Soil Solid Phase and Soil Solution (Grif i K, Tula, 2007) [in Russian].
E. A. Taraban, O. P. Krivoruchko, L. M. Plyasova, I. P. Olen’kova, and R. A. Buyanov, “Co-precipitated Fe(III)–Al(III) hydroxides: formation and crystallization during aging,” Izv. Sib. Otd. Nauk SSSR, Ser. Khim. Nauk, No. 1, 10–15 (1990).
E. V. Shamrikova, E. V. Vanchikova, T. A. Sokolova, E. V. Zhangurov, S. V. Deneva, Yu. I. Bobrova, and E. V. Kyzyurova, “Potential sources of exchangeable acidity in strongly acid soils (pHKCl < 3.3) and validation of its determination,” Eurasian Soil Sci. 51 (12), 1397–1410 (2018). https://doi.org/10.1134/S1064229318120116
E. V. Shamrikova, S. V. Deneva, O. S. Kubik, V. V. Punegov, E. V. Kyzyurova, Yu. I. Bobrova, and O. M. Zueva, “Acidity in organic horizons of arctic soils on the Barents Sea coast,” Eurasian Soil Sci. 50 (11), 1283–1293 (2017). https://doi.org/10.1134/S1064229317110102
E. V. Shamrikova, S. V. Deneva, A. N. Panyukov, and O. S. Kubik, “Soils and vegetation of the Khaipudyr Bay coast of the Barents Sea,” Eurasian Soil Sci. 51 (4), 385–394 (2018). https://doi.org/10.1134/S1064229318040129
A. Delgado, F. González-Caballero, and J. Bruque, “On the zeta potential and surface charge density of montmorillonite in aqueous electrolyte solutions,” Colloid Interface Sci. 113, 203–211 (1986).
M. Kosmulski, “Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review years after Parks’ review,” Adv. Colloid Interface Sci. 238, 1–61 (2016).
U. Schwertmann and R. M. Comell, Iron Oxides in the Laboratory: Preparation and Characterization (Wiley, Chichester, 1991).
The Environmental Chemistry of Aluminum, Ed. by G. Sposito (CRC Press, Boca Raton, 1996).
M. A. Torlopov, V. I. Mikhaylov, E. V. Udoratina, L. A. Aleshina, A. I. Prusskii, N. V. Tsvetkov, and P. V. Krivoshapkin, “Cellulose nanocrystals with different length-to-diameter ratios extracted from various plants using novel system acetic acid/phosphotungstic acid/octanol-1,” Cellulose 25, 1031–1046 (2018).
X. Zhu, H. Chen, W. Li, Y. He, P. C. Brookes, R. White, and J. M. Xu, “Evaluation of the stability of soil nanoparticles: the effect of natural organic matter in electrolyte solutions,” Eur. J. Soil Sci. 68, 105–114 (2017).
M. A. Wells, R. W. Fitzpatrick, and R. J. Gilkes, “Thermal and mineral properties of Al-, Cr-, Mn-, Ni- and Ti-substituted goethite,” Clays Clay Miner. 54 (2), 176–194 (2006).
ACKNOWLEDGMENTS
We are grateful to Candidate of Biology S.V. Deneva and Candidate of Agriculture E.V. Zhangurov (Institute of Biology, Komi Research Center, Ural Branch of the Russian Academy of Sciences) for presented information about soils and valuable advice.
Funding
This study was performed within the framework of the state assignment on the assessment of peat soils in the Arctic and Subarctic regions of the northeast of European Russia (registration no. АААА-А17-117122290011-5) and was partly supported by the Russian Foundation for Basic Research, project no. 20-04-00445a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by T. Chicheva
Rights and permissions
About this article
Cite this article
Vanchikova, E.V., Shamrikova, E.V., Korolev, M.A. et al. Application of Model Systems Containing Exchangeable Iron(III) to Study Acidity Characteristics of Strongly Acid Soils (pHKCl < 3.3). Eurasian Soil Sc. 54, 189–200 (2021). https://doi.org/10.1134/S1064229321020150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229321020150