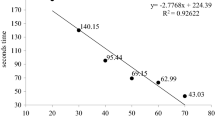
Abstract
Data on the functional aspects of the effect of more than 25 types of anesthetic chemicals and physical factors on teleostean fishes are generalized. It is shown that most of the narcotic agents have a pronounced stress effect on the fish and radically change the fish state. Many of these agents cause a functional response at the level of separate physiological systems (respiration, blood circulation, and blood). It is concluded that the use of anesthetics should be differentiated in the practice of scientific research. For example, the use of isoeugenol (AQUI-S) has no effect on the level of cortisol and catecholamines in the blood. Propanidide and urethane are neutral agents with respect to the respiratory and circulatory systems. Alflaxalone and ketamine do not affect the cardiac rhythm. The hematological parameters have no statistically significant changes under the effect of thiopental. The processes of carbohydrate metabolism in fish tissues are insensitive to the following anesthetic agents: isoeugenol (AQUI-S), urethane, hydrochloride, and a combination of quinaldine with a muscle relaxant (diazepam). An analysis of the stages of narcotic state development in the fish has revealed the advantage of using urethane, hydrochloride, clove oil, and AQUI-S. These types of anesthesia are not characterized by the development of the excitement state; the individuals of many species immediately turn to the resting stage (loss of pain sensitivity), which is most suitable for carrying out manipulations with fish. This information makes it possible to reduce incidental functional effects that can be caused by manipulation measures or by the use of separate narcotic agents. This allows one to obtain more reliable results, especially during experimental works.
Similar content being viewed by others
REFERENCES
Akbulut, B., Çakmak, E., Aksungur, N., and Çavdar, Y., Effect of exposure duration on time to recovery from anaesthesia of clove oil in juvenile of Russian sturgeon, Turk. J. Fish. Aquat. Sci., 2011, vol. 11, p. 463. https://doi.org/10.4194/1303-2712-v11_3_17
Anderson, W.G., McKinley, R.S., and Colavecchia, M., The use of clove oil as an anesthetic for rainbow trout and its effects on swimming performance, North Am. J. Fish. Manage., 1997, vol. 17, no. 2, p. 301. https://doi.org/10.1577/1548-8675(1997)017<0301:TUOCOA>2.3.CO;2
Barham, W.T. and Schoonbee, H.J., A comparison of the effects of alternating current electronarcosis, rectified current electronarcosis and chemical anaesthesia on the blood physiology of the freshwater bream Oreochromis mossambicus (Peters). I. The effect on blood pH, pO2, pCO2, glucose, lactate, LDH and HBDH, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1990, vol. 96, no. 2, p. 333.
Barham, W.T. and Schoonbee, H.J., A comparison of the effects of alternating current electronarcosis, rectified current electronarcosis and chemical anaesthesia on the blood physiology of the freshwater bream Oreochromis mossambicus (Peters). 2. The effect on haematocrit, haemoglobin concentration, red cell count, mean cell volume, mean cell haemoglobin and mean cell haemoglobin concentration, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1991, vol. 98, no. 2, p. 179.
Barham, W.T., Schoonbee, H.J., Visser, J.G.J., and Smit, G.L., A comparison of red-cell fragilities of electronarcotized and chemically anaesthetized freshwater bream, Oreochromis mossambicus, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1988, vol. 91, no. 2, p. 241.
Barton, B.A., Salmonid fishes differ in their cortisol and glucose responses to handling and transport stress, North Am. J. Aquacult., 2000, vol. 62, p. 12. https://doi.org/10.1577/1548-8454(2000)062<0012:SFDITC>2.0.CO;2
Barton, B.A. and Peter, R.E., Plasma cortisol stress response in fingerling rainbow trout, Salmo gairdneri Richardson, to various transport conditions, anaesthesia, and cold shock, J. Fish. Biol., 2006, vol. 20, no. 1, p. 39. https://doi.org/10.1111/j.1095-8649.1982.tb03893.x
Barton, B.A., Rahn, A.B., Feist, G., et al., Physiological stress responses of the freshwater chondrostean paddlefish (Polyodon spathula) to acute physical disturbances, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1998, vol. 120, p. 355. https://doi.org/10.1016/S1095-6433(98)10036-3
Braley, H. and Anderson, T.A., Changes in blood metabolite concentrations in response to repeated capture, anaesthesia and blood sampling in the golden perch, Macquaria ambigua, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol.,1992, vol. 103, no. 3, p. 445.
Burleson, M.L. and Smatresk, N.J., The effect of decerebration and anesthesia on the reflex responses to hypoxia in catfish, Can. J. Zool., 1989, vol. 67, no. 3, p. 630.
Carragher, J.F. and Rees, C.M., Primary and secondary stress responses in golden perch, Macquaria ambigua, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., vol. 107, no. 1, p. 49.
Chance, R.J., Cameron, G.A., Fordyce, M., et al., Effects of repeated anaesthesia on gill and general health of Atlantic Salmon, Salmo salar, J. Fish. Biol., 1994, vol. 93, no. 6, p. 1069. https://doi.org/10.1111/jfb.13803
Chiba, A. and Chichibu, S., High-energy phosphate metabolism in the phenthiazamine hydrobromide anesthetized loach Cobitis biwae, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1992, vol. 102, no. 3, p. 433.
Chiba, A. and Chichibu, S., High-energy phosphate metabolites in loach (Cobitis biwae) during urethane anesthesia, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1993, vol. 106, no. 1, p. 87.
Chiba, A., Hamaguchi, M., Kosaka, M., et al., In vivo 31P-NMR analysis of the electric anesthetized loach, Cobitis biswae, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1990a, vol. 97, no. 3, p. 385.
Chiba, A., Hamaguchi, M., Kosaka, M., et al., Energy metabolism in unrestrained fish with in vivo 31P-NMR, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1990b, vol. 96, no. 2, p. 253.
Chiba, A., Hamaguchi, M., Tokuno, T., et al., Changes in high-energy phosphate metabolites in loaches (Cobitis biwae) during 2-phenoxyethanol anesthesia, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1990c, vol. 97, no. 1, p. 183.
Cooper, A.R. and Morris, S., The blood respiratory, haematological, acid-base and ionic status of the Port Jackson shark, Heterodontus portusjacksoni, during recovery from anaesthesia and surgery: a comparison with sampling by direct caudal puncture, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1998, vol. 119, no. 4, p. 895.
Cornish, I.M.E. and Moon, T.W., The glucose and lactate kinetics of American eels, Anguilla rostrata (LeSueur), under MS 222 anaesthesia, J. Fish. Biol., 1986, vol. 28, no. 1, p. 1.
D’yakonov, Yu.N., Influence of anesthetic 2-methyl-4-vinyloxyquinoline hydrochloride on respiration and cardiac activity of carp, Sb. Nauch. Tr. Nauchno-Issled. Inst. Ozern. Rechn. Rybn. Khoz., 1980, no. 157, p. 50.
Davidson, G., Davie, P.S., Young, G., and Fowler, R.T., Physiological responses of rainbow trout Oncorhynchus mykiss to crowding and anesthesia with AQUI-S™, J. World Aquacult. Soc., 2000, vol. 31, no. 1, p. 105. https://doi.org/10.1111/j.1749-7345.2000.tb00704.x
Davis, K.B. and Griffin, B.R., Physiological responses of hybrid striped bass under sedation by several anesthetics, Aquaculture, 2004, vol. 233, no. 1. https://doi.org/10.1016/j.aquaculture.2003.09.018
Deacon, N., White, H., and Hecht, T., Isolation of the effective concentration of 2-phenoxyethanol for anaesthesia in the spotted grunter, Pomadasys commersonnii, and its effect on growth, Aquarium Sci. Conserv., 1997, vol. 1, no. 1, p. 19.
Epple, A., Navarro, I., Horak, P., and Spector, S., Endogenous morphine and codeine: release by the chromaffin cells of the eel, Life Sci., 1993, vol. 52, no. 16, p. PL117.
Ferreira, J.T., Schoonbee, H.J., and Smit, G.L., The uptake of the anaesthetic benzocaine hydrochloride by the gills and skin of three freshwater fish species, J. Fish. Biol., 2006, vol. 25, no. 1, p. 35. https://doi.org/10.1111/j.1095-8649.1984.tb04848.x
Gabryelak, T., Zalesna, G., Roche, H., and Peres, G., The effect of MS-222 an anaestetics on the peroxide metabolism enzymes in erythrocytes of freshwater and marine fish species, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1989, vol. 92, no. 1, p. 5.
Githuria, C.M., Kembenya, E.M., and Opiyo, M.A., Anaesthetic effects of sodium bicarbonate at different concentrations on African catfish (Clarias gariepinus) juveniles, J. Aquacult. Eng. Fish. Res., 2016, vol. 2, no. 3, p. 151. https://doi.org/10.3153/JAEFR16017
Groettum, J.A., Erikson, U., Grasdalen, H., and Staurnes, M., In vivo 31P-NMR spectroscopy and respiration measurements of anaesthetized goby (Pomatoschistus sp.) pre-exposed to ammonia, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1998, vol. 120, no. 3, p. 469.
Handa, T., Namba, K., Uematsu, K., and Yoshida, M., Blood properties and cardiovascular function after the cannulation into the dorsal aorta in carp, Cyprinus carpio, Appl. Biol. Sci., 1996, vol. 35, no. 2, p. 139.
Hansen, M.K., Nymoen, U., and Horsberg, T.E., Pharmokinetic and pharmacodynamic properties of metomidate in turbot (Scophthalmus maximus) and halibut (Hippoglossus hippoglossus), J. Vet. Pharmacol. Ther., vol. 26, no. 2, p. 95. https://doi.org/10.1046/j.1365-2885.2003.00454.x
Harms, C.A., Lewbart, G., Swanson, C.R., and Boylan, S.M., Behavioral and clinical pathology changes in koi carp (Cyprinus carpio) subjected to anesthesia and surgery with and without intra-operative analgesics, Comp. Med., 2005, vol. 55, no. 3, p. 221.
Haux, C., Sjobeck, M.L., and Larsson, A., Physiological stress responses in a wild fish population of perch (Perca fluviatilis) after capture and during subsequent recovery, Mar. Environ. Res., 1985, vol. 15, no. 2, p. 77.
Hedrick, M.S. and Winmill, R.E., Excitatory and inhibitory effects of tricaine (MS-222) on fictive breathing in isolated bullfrog brain stem, AJP Regul. Integr. Comp. Physiol., 2003, vol. 284, no. 2, p. R405. https://doi.org/10.1152/ajpregu.00418.2002
Hikasa, Y., Anesthesia and recovery with tricaine methanesulfonate, eugenol and thiopental sodium in the carp, Cyprinus carpio, Jpn. J. Vet. Sci., 1986, vol. 48, no. 2, p. 341.
Hill, J.V. and Forster, M.E., Cardiovascular responses of Chinook Salmon (Oncorhynchus tshawytscha) during rapid anesthetic induction and recovery, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2004, vol. 137, no. 2, p. 167. https://doi.org/10.1016/j.cca.2004.01.002
Hill, J.V., Davison, B., and Forster, M.E., The effects of fish anaesthetics (MS222, metomidate and AQUI-S) on heart ventricle, the cardiac vagus and branchial vessels from Chinook salmon (Oncorhynchus tshawytscha), Fish Physiol. Biochem., 2002, vol. 27, no. 1, p. 19. https://doi.org/10.1023/B:FISH.0000021742.30567.2d
Hoffman, R., Lommel, R., and Riedl, M., Influence of different anaesthetics and bleeding methods on hematological values in fish, Arch. Fischereiwiss., 1982, vol. 33, nos. 1–2, p. 91.
Holloway, A.C., Keene, J.L., Noakes, D.L.G., and Moccia, R.D., Effects of clove oil and MS-222 on blood hormone profiles in rainbow trout Oncorhynchus mykiss, Walbaum, Aquacult. Res., 2004, vol. 35, no. 11, p. 1025. https://doi.org/10.1111/j.1365-2109.2004.01108.x
Houston, A.H., Czerwinski, C.L., and Woods, R.J., Cardiovascular–respiratory activity during recovery from anesthesia and surgery in brook trout (Salvelinus fontinalis) and carp (Cyprinus carpio), J. Fish. Res. Board Can., 1973, vol. 30, no. 11, p. 1705.
Hseu, J.R., Yeh, Sh.L., Chu, Y.T., and Ting, Y.Y., The changes of hematological parameters during sustained anaesthesia with 2-phenoxyethanol in yellowfin porgy (Acanthopagrus latus), J. Taiwan Fish. Res., 1994, vol. 2, no. 2, p. 63.
Itazawa, Y. and Takeda, T., Respiration of carp under anesthesia induced by mixed bubbling of carbon dioxide and oxygen, Bull. Jpn. Soc. Sci. Fish., 1982, vol. 48, no. 4, p. 489.
Iwama, G.K., Mcgeer, J.C., and Pawluk, M.P., The effects of five fish anaesthetics on acid-base balance, hematocrit, blood gases, cortisol, and adrenaline in rainbow trout, Can. J. Zool., 2011, vol. 67, no. 8, p. 2065.
Javaheri, S. and Moradlu, A.H., AQUI-S, a new anesthetic for use in fish propagation, Global Vet., 2012, vol. 9, no. 2, p. 205. https://doi.org/10.5829/idosi.gv.2012.9.2.64167
Karlsson-Drangsholt, A., Rosseland, B.O., Massabuau, J-C., and Kiessling, A., Pre-anaesthetic metomidate sedation delays the stress response after caudal artery cannulation in Atlantic cod (Gadus morhua), Fish Physiol. Biochem., 2011, vol. 38, no. 2, p. 401.
Kazuñ, K. and Siwicki, A.K., Propiscin—a safe new anaesthetic for fish, Arch. Pol. Fish., 2012, vol. 20, p. 173. https://doi.org/10.2478/v10086-012-0021-3
Kiessling, A., Johansson, D., Zahl, I.H., and Samuelsen, O.B., Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration, Aquaculture, 2009, vol. 296, nos. 3–4, p. 301. https://doi.org/10.1016/j.aquaculture.2008.09.037
Kildea, M.A., Allan, G.L., and Kearney, R.E., Accumulation and clearance of the anaesthetics clove oil and AQUI-S™ from the edible tissue of silver perch (Bidyanus bidyanus), Aquaculture, 2004, vol. 232, nos. 1–4, p. 265. https://doi.org/10.1016/s0044-8486(03)00483-6
King, W., Hooper, B., Hillsgrove, S., and Berlinsky, D.L., The use of clove oil, metomidate, tricaine methanesulphonate and 2-phenoxyethanol for inducing anaesthesia and their effect on the cortisol stress response in black sea bass (Centropristis striata L.), Aquacult. Res., 2005, vol. 36, no. 14, p. 1442. https://doi.org/10.1111/j.1365-2109.2005.01365.x
Knoph, M.B., Effects of metomidate anaesthesia or transfer to pure sea water on plasma parameters in ammonia-exposed Atlantic Salmon (Salmo salar L.) in sea water, Fish Physiol. Biochem., 1995, vol. 14, no. 2, p. 103. https://doi.org/10.1007/BF00002454
Kohbarae, J., Nanda, K., and Murachi, S., The heart rate of carp anesthetized with tetraethylene glycol dibutyl etther, Bull. Jpn. Soc. Sci. Fish., 1987, vol. 53, no. 4, p. 681.
Korcock, D.E., Houston, A.H., and Gray, J.D., Effects of sampling conditions on selected blood variables of rainbow trout, Salmo gairdneri Richardson, J. Fish. Biol., 1988, vol. 33, no. 2, p. 319.
Korstrom, J.S., Birtwell, I.K., Piercey, G.E., et al., Effect of hypoxia, fresh water, anaesthesia and sampling technique on the hematocrit values of adult sockeye Salmon (Oncorhynchus nerka), Can. Tech. Rep. Fish. Aquat. Sci., 1996, p. 34.
Krejszeff, S., Żarski, D., Palińska-Żarska, K., et al., Procedure for harmless estimation of fish larvae weight, Ital. J. Anim. Sci., 2013, vol. 12, no. 44, p. 270. https://doi.org/10.4081/ijas.2013.e44
Kristan, J., Stara, A., Polgesek, M., et al., Efficacy of different anaesthetics for pikeperch (Sander lucioperca L.) in relation to water temperature, Neuroendocrinol. Lett., 2014, vol. 35, suppl. 2, p. 81.
Laidley, C.W. and Leatherland, J.F., Cohort sampling, anaesthesia and stocking-density effects on plasma cortisol, thyroid hormone, metabolite and ion levels in rainbow trout, Salmo gairdneri R., J. Fish. Biol., 1988, vol. 33, no. 1, p. 73.
Lambooij, B., Pilarczyk, M., Bialowas, H., and Van de Vis, H., Anaesthetic properties of propiscin (etomidaat) and 2-phenoxyethanol in the common carp (Cyprinus carpio L.), neural and behavioural measures, Aquacult. Res., 2009, vol. 40, no. 11, p. 1328. https://doi.org/10.1111/j.1365-2109.2009.02233.x
Limanskii, V.V. and Martem’yanov, V.I., Electrocardiographic determination of the degree of fish anesthesia, VI Vses. konf. po ekologicheskoi fiziologii i biokhimii ryb (sentyabr’, 1985), Tezisy dokladov (VI All-Union Conf. on Ecological Physiology and Biochemistry of Fishes, September 1985, Abstracts of Papers), Vilnius, 1985, p. 119.
Margaritov, N., Effect of the anesthetic tricaine methanesulfonate (MS-222) on the size and age composition of erythrocytes in carp peripheral blood, Sof. Univ. Biol. Fak. Zool., 1984, vol. 75, no. 1, p. 71.
Martins, T., Valentim, A., Pereira, N., and Antunes, L.M., Anaesthetics and analgesics used in adult fish for research: a review, Lab. Anim., 2019, vol. 53, no. 4, p. 325. https://doi.org/10.1177/0023677218815199
Marx, H., Brunner, B., Weinzierl, W., et al., Comparative investigations on different methods for stunning fish with special regard to meat quality parameters, in Proc. Conf. IIR Comm. C2, Paris: Ins. Inter. Du Froid, 1996, p. 199.
Medeiros Júnior, E.F., Uehara, S.A., et al., Effectiveness of benzocaine as anesthetic at different water temperatures for early juvenile curimba (Prochilodus lineatus valenciennes, 1836), a neotropical fish species, Bol. Inst. Pesca, 2019, vol. 45, no. 3, p. 474. https://doi.org/10.20950/1678-2305.2019.45.3.474
Mirosnichenko, O.R., Injection anesthesia of carp, Sb. Nauchn. Tr. Vses. Nauchno-Issled. Inst. Prud. Rybn. Khoz., 1990, no. 59, p. 163.
Morales, A.E., Cardenete, G., Abellan, E., and Garcia-Rejon, L., Stress-related physiological responses to handling in common dentex (Dentex dentex Linnaeus, 1758), Aquacult. Res., 2005, vol. 36, p. 33. https://doi.org/10.1111/j.1365-2109.2004.01180.x
Mylonas, C.C., Cardinaletti, G., Sigelaki, I., and Polzonetti-Magni, A., Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures, Aquaculture, 2005, vol. 246, no. 1, p. 467. https://doi.org/10.1016/j.aquaculture.2005.02.046
Neiffer, D. and Stamper, M.A., Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs, ILAR J, 2009, vol. 50, no. 4, p. 343. https://doi.org/10.1093/ilar.50.4.343
Oikari, A. and Soivio, A., Influence of sampling methods and anaesthetization on various haematological parameters of several teleosts, Aquaculture, 1975, vol. 6, no. 2, p. 171.
Ortuño, J., Esteban, M.A., and Meseguer, J., Effects of four anaesthetics on the innate immune response of gilthead seabream (Sparus aurata L.), Fish Shellfish Immunol., 2002, vol. 12, no. 1, p. 49. https://doi.org/10.1006/fsim.2001.0353
Oswald, R.L., Injection anaesthesia for experimental studies in fish, Comp. Biochem. Physiol., Part C: Comp. Pharmacol.,1978, vol. 60, no. 1, p. 19.
Parma de Croux, M.J. and Montagna, M., Efficacy of benzocaine as an anesthetic for juveniles Pimelodus clarias maculatus (Pisces, Pimelodidae), Iheringia Ser. Zool., 1998, no. 84, p. 29.
Peake, S., Sodium bicarbonate and clove oil as potential anesthetics for non-Salmonid fishes, North Am. J. Fish Manage., 1998, vol. 18, no. 4, p. 919. https://doi.org/10.1577/1548-8675(1998)018<0919:SBACOA>2.0.CO;2
Pearson, M.P. and Stevens, E.D., Size and hematological impact of the splenic erythrocyte reservoir in rainbow trout, Oncorhynchus mykiss, Fish Physiol. Biochem., 1991, vol. 9, no. 1, p. 39.
Popovic, N.T., Strunjak-Perovic, I., Coz-Rakovac, R., et al., Tricaine methane-sulfonate (MS-222) application in fish anaesthesia (review), J. Appl. Ichthyol., 2012, vol. 28, p. 553. https://doi.org/10.1111/j.1439-0426.2012.01950.x
Post, G., Carbonic acid anesthesia for aquatic organisms, Progr. Fish-Cult., 1979, vol. 41, no. 3, p. 142.
Purbosari, N., Warsikic, E., Syamsuc, K., and Santosod, J., Natural versus synthetic anesthetic for transport of live fish: a review, Aquacult. Fish., 2019, no. 4, p. 129.
Readman, G.D., Owen, S.F., Knowles, T.G., and Murrell, J.C., Species specific anaesthetics for fish anaesthesia and euthanasia, Sci. Rep., 2017, vol. 7, no. 1, p. 7102. https://doi.org/10.1038/s41598-017-06917-2
Regan, M.D., Turko, A.J., Heras, J., et al., Ambient CO2, fish behaviour and altered gabaergic neurotransmission: exploring the mechanism of CO2-altered behaviour by taking a hypercapnia dweller down to low CO2 levels, J. Exp. Biol., 2016, vol. 219, p. 109. https://doi.org/10.1242/jeb.131375
Roubach, R., De-Carvalho-Gomes, L., and Val, A.L., Safest level of tricaine methanesulfonate (MS-222) to induce anesthesia in juveniles of matrinxa, Brycon cephalus, Acta Amazon., 2001, vol. 31, no. 1, p. 159. https://doi.org/10.1590/1809-43922001311163
Rożyński, M., Demska-Zakęś, K., Sikora, A., and Zakęś, Z., Impact of inducing general anesthesia with propiscin (etomidate) on the physiology and health of European perch (Perca fluviatilis L.), Fish Physiol. Biochem., 2018, vol. 44, no. 3, p. 927. https://doi.org/10.1007/s10695-018-0482-4
Ryan, S., The dynamics of MS-222 anaesthesia in a marine teleost (Pagrus auratus: Sparidae), Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1992, vol. 101, no. 3, p. 593.
Ryan, S.N., Davie, P.S., Gesser, H., and Wells, R.M.G., The effect of MS-222 on paced ventricle strips and the perfused heart of rainbow trout, Oncorhyncus mykiss, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1993, vol. 106, no. 2, p. 549.
Sandodden, R., Finstad, B., and Iversen, M., Transport stress in Atlantic Salmon (Salmo salar): anaesthesia and recovery comparative physiology and biochemistry, Aquacult. Res., 2001, vol. 32, p. 987. https://doi.org/10.1046/j.1365-2109.2001.00533.x
Sherwani, F.A. and Parwez, I., Effects of stress and food deprivation on catfish, Heteropneustes fossilis (Bloch), Indian J. Exp. Biol., 2000, vol. 38, no. 4, p. 379.
Silbernagel, C. and Yochem, P., Effectiveness of the anesthetic AQUI-S® 20E in marine finfish and elasmobranchs, J. Wild. Dis., 2016, vol. 52, no. 2, pp. S96–S103. https://doi.org/10.7589/52.2S.S96
Simões, L.N., Lombardi, D.C., Gomide, A.T.M., and Gomes, L.C., Efficacy of clove oil as anesthetic in handling and transportation of Nile tilapia, Oreochromis niloticus (Actinopterygii: Cichlidae) juveniles, Zoologia, 2011, vol. 28, no. 3, p. 285. https://doi.org/10.1590/S1984-46702011000300001
iwicki, A., Znieczulenie ogolne u ryb. Czesc I. Nowy preparat do znieczulenia ogolnego ryb, Gosp. Ryb., 1983, vol. 35, no. 11, p. 5
Small, B.C., Anesthetic efficacy of metomidate and comparison of plasma cortisol responses to tricaine methanesulfonate, quinaldine and clove oil anesthetized channel catfish Ictalurus punctatus, Aquaculture, 2003, vol. 218, nos. 1–4, p. 177. https://doi.org/10.1016/s0044-8486(02)00302-2
Small, B.C., Effect of isoeugenol sedation on plasma cortisol, glucose, and lactate dynamics in channel catfish Ictalurus punctatus exposed to three stressors, Aquaculture, 2004, vol. 238, nos. 1–4, p. 469. https://doi.org/10.1016/j.aquaculture.2004.05.021
Small, B.C. and Chatakondi, N.G., Routine measures of stress are reduced in mature channel catfish during and after aqui-s anesthesia and recovery, North Am. J. Aquacult., 2005, vol. 67, no. 1, p. 72.
Smith, M.F.L., Capture and transportation of elasmobranchs, with emphasis on the grey nurse shark (Carcharias taurus), Aust. J. Mar. Freshwater Res., 1992, vol. 43, no. 1, p. 325.
Smit, G.L., Hattingh, J., and Burger, A.P., Haematological assessment of the effects of the anaesthetic MS-222 in natural and neutralized form in three freshwater fish species: haemoglobin electrophoresis, ATP levels and corpuscular fragility curves, J. Fish. Biol., 1979, vol. 15, no. 6, p. 655.
Soivio, A. and Hughes, G.M., Circulatory changes in secondary lamellae of Salmo gairdneri gills in hypoxia and anaesthesia, Ann. Zool. Fenn., 1978, vol. 15, no. 3, p. 221.
Soivio, A., Nyholm, K., and Huhti, M., Effects of anaesthesia with MS 222, neutralized MS 222 and benzocaine on the blood constituents of rainbow trout, Salmo gairdneri, J. Fish. Biol., 2006, vol. 10, no. 1, p. 91. https://doi.org/10.1111/j.1095-8649.1977.tb04045.x
Soldatov, A.A., Oxygen-dissociation properties of blood and intraerythrocytic medium composition in sea fish with different motor activity, J. Evol. Biochem. Physiol., 1997, vol. 33, no. 6, p. 534.
Soldatov, A.A., Physiological aspects of effects of urethane anesthesia on the organism of marine fishes, Hydrobiol. J., 2005, vol. 41, no. 1, p. 113. https://doi.org/10.1615/HydrobJ.v41.i1.130
Soldatov, A.A., Parfyonova, I.A., and Novitskaya, V.N., Contents of monovalent cations and atp in erythrocytes of marine fishes under experimental hypoxia, Ukr. Biokhim. Zh., 2010, vol. 82, no. 2, p. 36.
Soto, C., Clove oil: a fish anaesthetic, Window Newsl., 1995, vol. 6, no. 2, p. 2.
Spotte, S., Bubucis, P.M., and Anderson, G., Plasma cortisol response of seawater-adapted mummichogs (Fundulus heteroclitus) during deep MS-222 anesthesia, Zoo-Biol., 1991, vol. 10, no. 1, p. 75.
Strebkova, T.P., Influence of anesthetics (sodium thiopental) on physiological parameters of underyearlings of mirror carp, Vopr. Ikhtiol., 1972, vol. 12, no. 2, p. 397.
Sumpter, J.P., Ehe endocrinology of stress, in Fish Stress and Health in Aquaculture, Soc. Exp. Biol. Sem., Ser. 62, Cambridge: Cambridge Univ. Press, 1997, p. 95.
Takeda, T., Yamasaki, K., and Itazawa, Y., Effect of MS-222 on respiration and effectiveness of artificial gills irrigation by anaestetic solution in carp, Bull. Jpn. Soc. Sci. Fish., 1987, vol. 53, no. 10, p. 1701.
Thomas, P. and Robertson, L., Plasma cortisol and glucose stress responses of red drum (Sciaenops ocellatus) to handling and shallow water stressors and anesthesia with MS-222, quinaldine sulfate and metomidate, Aquaculture, 1991, vol. 96, no. 1, p. 69.
Trushenski, J.T., Bowker, J.D., Cooke, S.J., et al., Issues regarding the use of sedatives in fisheries and the need for immediate-release options, Trans. Am. Fish. Soc., 2013, vol. 142, no. 1, p. 156. https://doi.org/10.1080/00028487.2012.732651
Valentim, A.M., Félix, L.M., Carvalho, L., et al., A new anaesthetic protocol for adult zebrafish (Danio rerio): propofol combined with lidocaine, PLoS One, 2016, vol. 11, p. 1. https://doi.org/10.1371/journal.pone.0147747
Veenstra, R.S., Balon, E.K., and Flegler-Balon, Ch., Propanidid—a usefull anaesthetic for studying blood circulation in early development of fish, Can. J. Zool., 1987, vol. 65, no. 5, p. 1286.
Velisek, J., Stejskal, V., Kouřil, J., and Svobodová, Z., Comparison of the effects of four anaesthetics on biochemical blood profiles of perch, Aquacult. Res., 2009, vol. 40, no. 3, p. 354. https://doi.org/10.1111/j.1365-2109.2008.02102.x
Vijayan, M.M., Pereira, C., Grau, E.G., and Iwama, G.K., Metabolic responses associated with confinement stress in tilapia: the role of cortisol, Comp. Biochem. Physiol., Part C: Comp. Pharmacol., 1997, vol. 116, p. 89.
Wang, Y., Wilkie, M.P., Heigenhauser, G.J.F., and Wood, C.M., The analysis of metabolites in rainbow trout white muscle: a comparison of different sampling and processing methods, J. Fish. Biol., 1994, vol. 45, no. 5, p. 855. https://doi.org/10.1111/j.1095-8649.1994.tb00950.x
Weber, R.A., Peleteiro, J.B., Garcia-Martin, L.O.G., and Aldegunde, M., The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858), Aquaculture, 2009, vol. 288, nos. 1–2, p. 147. https://doi.org/10.1016/j.aquaculture.2008.11.024
Wendelaar Bonga, S.E., The stress response in fish, Physiol. Rev., 1997, vol. 77, p. 591. https://doi.org/10.1152/physrev.1997.77.3.591
Weyl, O.L., Kaiser, H., and Hecht, T., On the efficacy and mode of action of 2-phenoxyethanol as an anaesthetic for goldfish, Carassius auratus (L.), at different temperatures and concentrations, Aquacult. Res., 2008, vol. 27, no. 10, p. 757. https://doi.org/10.1046/j.1365-2109.1996.t01-1-00791.x
White, H.I., Hecht, T., and Potgieter, B., The effect of four anaesthetics on Haliotis midae and their suitability for application in commercial abalone culture, Aquaculture, 1996, vol. 140, nos. 1–2, p. 145.
Witeska, M., Dudyk, J., and Jarkiewicz, N., Haematological effects of 2-phenoxyethanol and etomidate in carp (Cyprinus carpio L.), Vet. Anaesth. Analg., 2015, vol. 42, no. 5, p. 537. https://doi.org/10.1111/vaa.12242
Woolsey, J., Holcomb, M., and Ingermann, R.L., Effect of temperature on clove oil anesthesia in steelhead fry, North Am. J. Aquacult., 2004, vol. 66, no. 1, p. 35. https://doi.org/10.1577/A03-008
Wright, S. and Forster, M.E., Anaesthetic effects on the hepatic portal vein and on the vascular resistance of the tail of the Chinook Salmon (Oncorhynchus tshawytscha), Fish Physiol. Biochem., 2005, vol. 31, no. 1, p. 11.
Yanar, M. and Kumlu, M., The anaesthetics effects of quinaldine sulphate and/or diazepam on sea bass (Dicentrarchus labrax) juveniles, Turk. J. Vet. Anim. Sci., vol. 25, no. 2, p. 185.
Yin, M., Batty, R.S., Franklin, C.E., and Johnston, I.A., The influence of temperature and activity on oxygen consumption of larval herring (Clupea harengus L.), Oceanol. Limnol. Sin., 1995, vol. 26, no. 3, p. 285.
Yokoyama, Y., Kawai, F., and Kanamori, M., Effect of cold CO2 anesthesia on postmortem levels of ATP-related compounds, pH, and glycogen in carp muscle, Bull. Jpn. Soc. Sci. Fish., 1993, vol. 59, no. 12, p. 2047.
Yokoyama, T., Azuma, Y., Sakaguchi, M., Kawai, F., and Kanamori, M., 31P NMR study of bioenergetic changes in carp muscle with cold-CO2 anethesia and non-destructive evaluation of freshness, Fish. Sci., 1996, vol. 62, no. 2, p. 267.
Yoshikawa, H., Yokoyama, Y., Ueno, S., and Mitsuda, H., Changes of blood gas in carp, Cyprinus carpio, anesthetized with carbon dioxide, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1991a, vol. 98, nos. 3–4, p. 431.
Yoshikawa, H., Yokoyama, Y., Ueno, S., and Mitsuda, H., Electroencephalographic spectral analysis in carp, Cyprinus carpio, anesthetized with high concentrations of carbon dioxide, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1991b, vol. 98, nos. 3-4, p. 437.
Zahl, I.H., Kiessling, A., Samuelsen, O.B., and Hansen, M.K., Anaesthesia of Atlantic cod (Gadus morhua)—effect of pre-anaesthetic sedation, and importance of body weight, temperature and stress, Aquaculture, 2009, vol. 95, nos. 1–2, p. 52. https://doi.org/10.1016/j.aquaculture.2009.06.019
Zahl, I.H., Kiessling, A., Samuelsen, O.B., and Hansen, M.K., Anaesthesia of Atlantic halibut (Hippoglossus hippoglosus)—effect of pre-anaesthetic sedation and importance of body weight and water temperature, Aquacult. Res., 2010a, vol. 42, no. 9, p. 1235. https://doi.org/10.1111/j.1365-2109.2010.02711.x
Zahl, I.H., Kiessling, A., Samuelsen, O.B., and Olsen, R.E., Anesthesia induces stress in Atlantic Salmon (Salmo salar), Atlantic cod (Gadus morhua) and Atlantic halibut (Hippoglossus hippoglossus), Fish Physiol. Biochem., 2010b, vol. 36, no. 3, p. 719. https://doi.org/10.1007/s10695-009-9346-2
Zahl, I.H., Samuelsen, O.B., and Kiessling, A., Anesthesia of farmed fish: implications for welfare, Fish Physiol. Biochem., 2011, vol. 38, no. 1, p. 201. https://doi.org/10.1007/s10695-011-9565-1
Funding
This study was supported by state topic no. AAAA-A18-118021490093-4 and partially supported by the Russian Foundation for Basic Research, project no. 20-44-920001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by D. Zabolotny
Abbreviations: AQUI-S, isoeugenol; MS-222, sodium tricaine methanesulfonate.
Rights and permissions
About this article
Cite this article
Soldatov, A.A. Functional Effects of the Use of Anesthetics on Teleostean Fishes (Review). Inland Water Biol 14, 67–77 (2021). https://doi.org/10.1134/S1995082920060139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082920060139