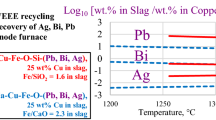
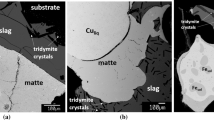
Abstract
A combination of high-temperature experiments and thermodynamic modeling in the CaO–Cu2O–FeO–Fe2O3–SiO2 system provides the fundamental information necessary to design and improve processes such as WEEE recycling through black copper route, continuous converting of copper matte, copper dross smelting, slag cleaning, and valorization of copper slag. In the experiments, samples were equilibrated on a primary phase substrate or Fe metal foil, then rapidly quenched and studied using the Electron Probe X-ray Microanalysis. The FactSage software was used for calculations, combined with custom developed thermodynamic database. A systematic analysis is provided to select the fluxing strategy and increase the copper recovery in industrial processes. Slag liquidus and concentration of copper in slag were assessed as functions of oxygen partial pressure, temperature, Fe/SiO2 in slag, and wt% CaO in slag.
Graphical Abstract









Similar content being viewed by others
References
Wood J, Matusewicz R, Reuter MA (2009) Ausmelt C3 converting. In: Kapusta J, Warner T (eds) International Peirce-Smith converting centennial. The Minerals, Metals & Materials Society, Warrendale, pp 397–406
Nikolic S, Shishin D, Hayes PC, Jak E (2018) Case study on the application of research to operations—calcium ferrite slags. In: Extraction 2018: Proceedings of the first global conference on extractive metallurgy, The Minerals, Metals & Materials Series. Springer, Cham, pp 391–402. https://doi.org/10.1007/978-3-319-95022-8_31
Kaur R, Nexhip C, Krippner D, George-Kennedy D, Routledge M (2011) Kennecott-Outotec ‘double flash’ technology after 16 years. In: 13th international flash smelting congress, Zambia
Yazawa A, Kongoli F (2001) Liquidus surface of newly defined “ferrous calcium silicate slag” and its metallurgical implications. High Temp Mater Processes 20:201–207
Somerville M, Chen C, Alvear GR, Nikolic S (2016) Fluxing strategies for the direct to blister smelting of high silica and low iron copper concentrates. In: Reddy R, Chaubal P, Pistorius PC, Pal U (eds) Advances in molten slags, fluxes, and salts: Proceedings of the 10th international conference on molten slags, fluxes and salts (MOLTEN16), Seattle, WA. The Minerals, Metals & Materials Society, pp 667–675
Klaffenbach E, Mostaghel S, Guo M, Blanpain B (2019) Evaluation of copper slag cleaning potentials. In: Proceedings of the 58th annual conference of metallurgists (COM) hosting the 10th international copper conference, CIM, Vancouver, Canada. Paper #594752
Wang J-P, Erdenebold U (2020) A study on reduction of copper smelting slag by carbon for recycling into metal values and cement raw material. Sustainability 12:1421. https://doi.org/10.3390/su12041421
Lykasov A, Ryss G, Sharafutdinov D, Pogodin A (2016) Extraction of iron from copper-plant slag. Steel Transl 46:609–613. https://doi.org/10.3103/S0967091216090059
Zhou X-L, Zhu D-Q, Jian P, Wu T-J (2015) Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel. ISIJ Int 55:1347–1352. https://doi.org/10.2355/isijinternational.55.1347
Bartie NJ, Abadias Llamas A, Heibeck M, Froehling M, Volkova O, Reuter MA (2020) The simulation-based analysis of the resource efficiency of the circular economy—the enabling role of metallurgical infrastructure. Miner Process Extr Metall 129:229–249. https://doi.org/10.1080/25726641.2019.1685243
Chen P, Xiao H, Chen J, Chen L, Zhang D, Liu W, Yang T (2020) Oxygen-rich side-blown bath smelting of copper dross: a process study. J Sustain Metall 6:344–354. https://doi.org/10.1007/s40831-020-00278-3
Ghodrat M, Rhamdhani MA, Khaliq A, Brooks G, Samali B (2018) Thermodynamic analysis of metals recycling out of waste printed circuit board through secondary copper smelting. J Mater Cycles Waste Manage 20:386–401. https://doi.org/10.1007/s10163-017-0590-8
Ghodrat M, Rhamdhani MA, Brooks G, Masood S, Corder G (2016) Techno economic analysis of electronic waste processing through black copper smelting route. J Clean Prod 126:178–190. https://doi.org/10.1016/j.jclepro.2016.03.033
Buchmann M, Borowski N, Leißner T, Heinig T, Reuter M, Friedrich B, Peuker U (2020) Evaluation of recyclability of a WEEE slag by means of Integrative X-ray computer tomography and SEM-based image analysis. Minerals 10:309. https://doi.org/10.3390/min10040309
Jak E, Hidayat T, Prostakova V, Shishin D, Shevchenko M, Hayes PC (2019) Integrated experimental and thermodynamic modelling research for primary and recycling pyrometallurgy. In: Paper presented at the EMC, Düsseldorf, Germany. Clausthal-Zellerfeld, Germany: GDMB Verlag, pp 587–604
Jak E, Hayes PC, Lee H-G (1995) Improved methodologies for the determination of high temperature phase equilibria. Korean J Miner Mater Inst (Seoul) 1:1–8. https://doi.org/10.1007/BF03055319
Jak E (2012) Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field. In: Paper presented at the 9th Intl. Conf. on Molten Slags, Fluxes and Salts (MOLTEN12), Beijing, China. The Chinese Society for Metals, pp 1–28
Shevchenko M, Jak E (2018) Experimental liquidus studies of the Pb-Fe-Si-O system in equilibrium with metallic Pb. Metall Mater Trans B 49:159–180. https://doi.org/10.1007/s11663-017-1136-0
Shevchenko M, Jak E (2019) Experimental liquidus studies of the Pb-Fe-Ca-O system in air. J Phase Equilib Diff 40:128–137. https://doi.org/10.1007/s11669-018-0703-7
Shevchenko M, Jak E (2019) Experimental liquidus studies of the binary Pb-Cu-O and ternary Pb-Cu-Si-O systems in equilibrium with metallic Pb-Cu alloys. J Phase Equilib Diff 40:671–685. https://doi.org/10.1007/s11669-019-00754-8
Kawasaki T, Ishizuka H (2008) Experimental study of Fe3+ solubility in cristobalite and its application to a metamorphosed quartz-magnetite rock from Mt. Riiser-Larsen area, Napier Complex, East Antarctica. J Mineral Petrol Sci 103:255–265. https://doi.org/10.2465/jmps.080216
Mohan K, Glasser FP (1977) The thermal decomposition of calcium silicate (Ca3SiO5) at temperatures below 1250 °C. I. Pure C3S and the influence of excess calcium oxide or calcium silicate (Ca2SiO4). Cem Concr Res 7:1–8
Hillert M, Sundman B, Wang X (1990) An assessment of the calcia-silica system. Metall Trans B 21B:303–312. https://doi.org/10.1016/j.jeurceramsoc.2016.01.046
Carlson ET (1931) Decomposition of tricalcium silicate. Rock Prod 34:52–56
Tromel G, Fix W, Heinke R (1969) High temperature investigations up to 1900 °C on calcium orthosilicate and trisilicate. Tonindustrie-Zeitung und Keramishe Rundschau 93:1–8
Eriksson G, Wu P, Blander M, Pelton AD (1994) Critical evaluation and optimisation of the thermodynamic properties and phase diagrams of the MnO-SiO2 and CaO-SiO2 systems. Can Metall Q 33:13–21. https://doi.org/10.1179/cmq.1994.33.1.13
Bale CW et al (2016) FactSage thermochemical software and databases, 2010–2016. CALPHAD 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Hidayat T, Jak E (2014) Thermodynamic modeling of the “Cu2O”-SiO2, “Cu2O”-CaO, and “Cu2O”-CaO-SiO2 systems in equilibrium with metallic copper. Int J Mater Res 105:249–257. https://doi.org/10.3139/146.111023
Hidayat T, Shishin D, Decterov SA, Jak E (2016) Critical thermodynamic re-evaluation and re-optimization of the CaO-FeO-Fe2O3-SiO2 system. CALPHAD 56:58–71. https://doi.org/10.1016/j.calphad.2016.11.009
Hidayat T, Shishin D, Decterov S, Jak E (2017) Critical assessment and thermodynamic modeling of the Cu-Fe-O-Si system. CALPHAD 58:101–114. https://doi.org/10.1016/j.calphad.2017.06.003
Shishin D, Hidayat T, Jak E (2019) Thermodynamic assessment of the CaO-Cu2O-FeO-Fe2O3 system. CALPHAD 68:101715. https://doi.org/10.1016/j.calphad.2019.101715
Shevchenko M, Jak E (2020) Thermodynamic optimization of the binary PbO-CaO and ternary PbO-CaO-SiO2 systems. Calphad. https://doi.org/10.1016/j.calphad.2020.101807
Pelton AD, Decterov SA, Eriksson G, Robelin C, Dessureault Y (2000) the modified quasichemical model. I—binary solutions. Metall Mater Trans B 31:651–659. https://doi.org/10.1007/s11663-000-0103-2
Pelton AD, Chartrand P (2001) The modified quasichemical model. II—multicomponent solutions. Metall Mater Trans A 32:1355–1360. https://doi.org/10.1007/s11661-001-0226-3
Hillert M (2001) The compound energy formalism. J Alloys Compd 320:161–176
Redlich O, Kister AT (1948) Thermodynamics of nonelectrolytic solutions. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Ing Chem 40:345–348
Hidayat T, Shishin D, Jak E, Decterov S (2015) Thermodynamic reevaluation of the Fe-O system. CALPHAD 48:131–144. https://doi.org/10.1016/j.calphad.2014.12.005
Hidayat T, Shishin D, Decterov SA, Jak E (2016) Thermodynamic optimization of the Ca-Fe-O system. Metall Trans B 47:256–281. https://doi.org/10.1007/s11663-015-0501-0
Hidayat T, Shishin D, Decterov SA, Jak E (2017) Experimental study and thermodynamic re-evaluation of the FeO-Fe2O3-SiO2 system. J Phase Equilib Diffus 38:477–492. https://doi.org/10.1007/s11669-017-0535-x
Shishin D, Hidayat T, Jak E, Decterov S (2013) Critical assessment and thermodynamic modeling of Cu-Fe-O system. CALPHAD 41:160–179. https://doi.org/10.1016/j.calphad.2013.04.001
Shishin D, Decterov SA (2012) Critical assessment and thermodynamic modeling of Cu-O and Cu-O-S systems. CALPHAD 38:59–70. https://doi.org/10.1016/j.calphad.2013.04.001
Eriksson G, Pelton AD (1993) Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 systems. Metall Trans 24:807–816. https://doi.org/10.1111/j.1151-2916.1993.tb08334.x
Hellstén N, Klemettinen L, Sukhomlinov D, O’Brien H, Taskinen P, Jokilaakso A, Salminen J (2019) Slag cleaning equilibria in iron silicate slag-copper systems. J Sustain Metall 5:463–473. https://doi.org/10.1007/s40831-019-00237-7
Henao HM, Hayes PC, Jak E (2012) Phase equilibria of "Cu2O"-"FeO"-SiO2-CaO slags at PO2 at 10–8 atm in equilibrium with metallic copper. In: Ninth international conference on molten slags, fluxes and salts (MOLTEN12). The Chinese Society for Metals, Beijing, China, Paper W079
Nikolic S, Hayes PC, Jak E (2008) Phase equilibria in ferrous calcium silicate slags: part III. Copper-saturated slag at 1250 °C and 1300 °C at an oxygen partial pressure of 10–6 atm. Metall Trans B 39B:200–209. https://doi.org/10.1007/s11663-008-9128-8
Jak E, Hidayat T, Shishin D, Mackey PJ, Hayes PC (2018) Modelling of liquid phases and metal distributions in copper converters: transferring process fundamentals to plant practice. Miner Process Extr Metall. https://doi.org/10.1080/25726641.2018.1506273
Jak E, Nexhip C, George-Kennedy DP (2014) Calcium ferrite slag phase chemistry control used in continuous flash converting. In: Ed. R. Bassa, R. Parra, A. Luraschi, and S. Demetrio, Proceedings of Copper 2013, International Copper Conference, the Nickolas Themelis Symposium on Pyrometallurgy and Process Engineering, The Chilean Institute of Mining Engineers, Santiago, Chile, vol. III (book 1), pp 503–522
Jak E, Zhao B, Nexhip C, George-Kennedy DP, Hayes PC (2010) Liquidus temperature in calcium ferrite slags in Ca2Fe2O5 and Ca2SiO4 primary phase fields with Cu and fixed PO2. In: Proceedings of copper 2010 conference, Hamburg, Germany, GDMB, pp 811–821
Acknowledgements
The authors acknowledge the financial support and technical guidance by the consortium of copper producers: Aurubis AG (Germany), BHP Billiton Olympic Dam Operation (Australia), Glencore Technology (Australia), Outotec Oy (Finland), Anglo American Platinum (South Africa), Umicore NV (Belgium), Rio Tinto Kennecott (USA), Peñoles (Mexico), RHI-Magnesita (Austria), Boliden (Sweden), and Australian Research Council through Linkage project LP190101020 “Future copper metallurgy for the age of e-mobility and the circular economy.” The authors are grateful to Prof. Peter C. Hayes for discussions and valuable advice on this study and to Ms. Suping Huang for assistance with conducting experiments. Present study would not be possible without the facilities and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis in The University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Markus Reuter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shishin, D., Shevchenko, M. & Jak, E. Experimental Study and Thermodynamic Calculations in the CaO–Cu2O–FeO–Fe2O3–SiO2 System for Applications in Novel Copper-Based Processes. J. Sustain. Metall. 7, 300–313 (2021). https://doi.org/10.1007/s40831-021-00341-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00341-7