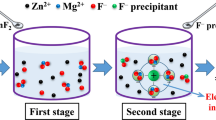
Abstract
This paper presents the hydrometallurgical methods used for the recovery of concentrate and metallic lead from zinc plant residue (ZPR). The ZPR mainly consisting of 9.5% Pb and 7.5% Zn was initially leached by sulfuric acid under the following conditions: leaching temperature 90 °C, leaching time 90 min., sulfuric acid concentration 200 g/l, and solid/liquid ratio 1/5. The zinc recovery obtained was 92.8%, which was finally directed to solvent extraction circuit to produce zinc cathode. Then acidic residue was subjected to alkaline leaching, using sodium hydroxide (NaOH). The lead recovery obtained was 92.58%. For precipitation method by using NaClO, the effects of three important factors including (NaClO/solution) ratio, temperature, and precipitation time were studied and reported. As a result, lead was precipitated in the form of PbO2 with concentrated grade of 72.03% and recovery of 92.31%. Furthermore, aluminum was used for cementation process to produce metallic Pb with grade of 96.11% and total recovery of 87.17%.
Graphical Abstract











Similar content being viewed by others
References
Shafaei SZ (1999) Hydrometallurgy. Shahrood University, Semnan
Evans D, Meddings B (1971) The changing role of Hydrometallurgy. Can Min Metall Bull 64(706):48–57
Habashi F (1969) Principles of extractive metallurgy, vol 1. CRC Press, Boca Raton
Raghavan R, Mohanan P, Swarnkar S (2000) Hydrometallurgical processing of lead-bearing materials for the recovery of lead and silver as lead concentrate and lead metal. Hydrometallurgy 58(2):103–116
Farahmand F et al (2009) Optimization and kinetics of the cementation of lead with aluminum powder. Hydrometallurgy 98(1):81–85
Turan MD, Altundoğan HS, Tümen F (2004) Recovery of zinc and lead from zinc plant residue. Hydrometallurgy 75(1):169–176
Liao M, Deng T (2004) Zinc and lead extraction from complex raw sulfides by sequential bioleaching and acidic brine leach. Miner Eng 17(1):17–22
Woo YT (1988) Natural, metal, fiber, and macromolecular carcinogens. Academic Press
Sahoo P, Rath P (1988) Recovery of lead from complex sulphide leach residue by cementation with iron. Hydrometallurgy 20(2):169–177
Ku Y, Lee C-S (1997) Kinetic study on the removal of lead from wastewaters by iron cementation. Journal of the Chinese Institute of Engineers 20(3):295–301
Habashi F (1999) A textbook of hydrometallurgy. Métallurgie Extractive Québec, Quebec
Dönmez B, Sevim F, Sarac H (1999) A kinetic study of the cementation of copper from sulphate solutions onto a rotating aluminum disc. Hydrometallurgy 53(2):145–154
Khudenko BM (1987) Mathematical models of cementation processes. J Environ Eng 113(4):681–702
Rashchi F, Sui C, Finch JA (2002) Sphalerite activation and surface Pb ion concentration. Int J Miner Process 67(1):43–58
Raghavan R, Mohanan P, Patnaik S (1998) Innovative processing technique to produce zinc concentrate from zinc leach residue with simultaneous recovery of lead and silver. Hydrometallurgy 48(2):225–237
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. The Authors: Behrouz Taheri, Mahdi Gharabaghi, and Sajjad Aghazadeh.
Additional information
The contributing editor for this article was T. Hirato.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taheri, B., Gharabaghi, M. & Aghazadeh, S. Pb Recycling Through Leaching, Precipitation, and Cementation from Zinc Plant Residue. J. Sustain. Metall. 7, 291–299 (2021). https://doi.org/10.1007/s40831-021-00338-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00338-2